DC (dendritic cell) inducer and application thereof
An inducer, GM-CSF technology, applied in cell culture active agents, microorganisms, blood/immune system cells, etc., can solve the problems of low induction maturation rate, low cell proliferation rate, poor antigen presentation performance, etc. Cell viability, increase the proliferation rate and maturation rate, and improve the effect of antigen presentation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The application of the DC inducer provided by the present invention in the method for inducing DC maturation comprises the following steps:
[0033] S1a, obtaining dendritic cell precursor mononuclear cells;
[0034] Draw 3-5ml of peripheral venous blood, dilute it with normal saline 1:1, then slowly add it to 3-5ml of Ficoll-Hypaque lymphocyte separation medium with a specific gravity of 1.077±0.001g / ml along the tube wall, and centrifuge at 1500r / min for 15 minutes , isolate and obtain peripheral blood mononuclear cells, and suspend the cells in RPMI1640 medium (purchased from Gibco) containing 10% fetal bovine serum, 100 U / mL penicillin, and 100 U / mL streptomycin at a concentration of 4×10 6 / ml, transferred to a six-well plate, 2ml / well, put the six-well plate at 37°C, 5% CO 2 Incubate in an incubator for 2 hours, remove the medium and suspended cells, put fresh RPMI1640 medium in each well, blow gently to blow up the cells on the wall, and collect the cell suspens...
Embodiment 2
[0042] The difference from Embodiment 1 is that in this embodiment,
[0043] Among the first inducers used,
[0044] The concentration of rhGM-CSF is 800U / ml,
[0045] The concentration of GM-CSF is 5ng / mL,
[0046] The concentration of IL-4 is 5ng / mL,
[0047] The concentration of rhIL-4 is 800U / ml,
[0048] and IL-13 at a concentration of 5 ng / mL;
[0049] In the second inducer,
[0050] The concentration of rhTNF-α is 800U / ml,
[0051] The concentration of gallic acid was 5 μg / ml,
[0052] Or the concentration of walnut green husk extract is 10μg / ml.
Embodiment 3
[0054] The difference from Embodiment 1 is that in this embodiment,
[0055] Among the first inducers used,
[0056] The concentration of rhGM-CSF is 1200U / ml,
[0057] The concentration of GM-CSF is 15ng / mL,
[0058] The concentration of IL-4 is 15ng / mL,
[0059] The concentration of rhIL-4 is 1200U / ml,
[0060] and IL-13 at a concentration of 15 ng / mL;
[0061] In the second inducer,
[0062] The concentration of rhTNF-α is 1200U / ml,
[0063] The concentration of gallic acid is 15μg / ml,
[0064] Or the concentration of walnut green bark extract is 30μg / ml.
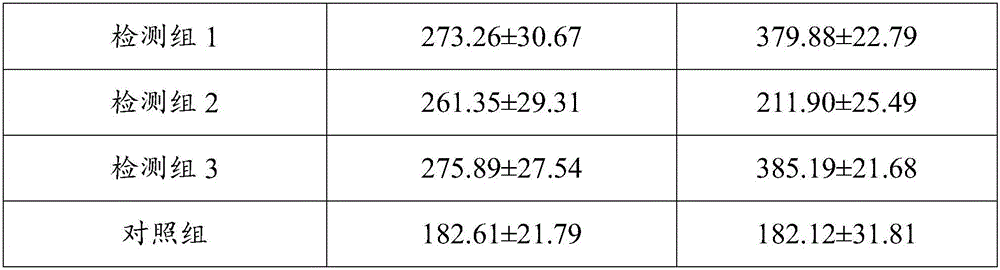
[0065] In order to further verify that the DC inducer provided by the present invention and the obtained dendritic cells have significant beneficial effects, the following experiments are set up for verification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com