Method for in-vitro isolated culture of chicken fallopian tube epithelial cells
A technology for the isolation and cultivation of epithelial cells, applied in the direction of cell culture active agents, artificial cell constructs, epidermal cells/skin cells, etc., can solve the problems of fibroblast interference, low cell acquisition rate, large cell damage, etc., and achieve digestion Short time, good effect, less miscellaneous cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
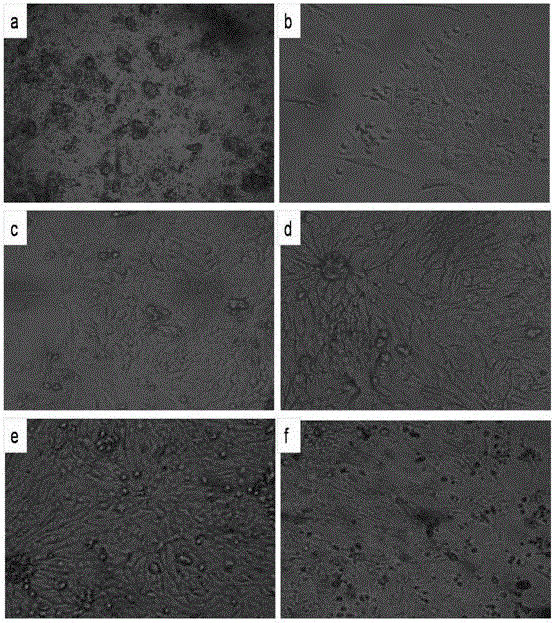
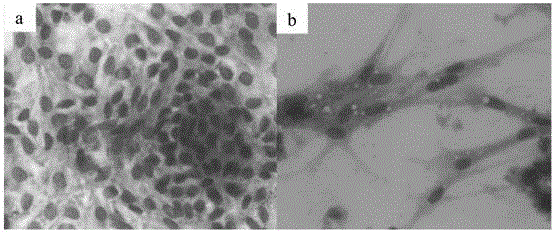
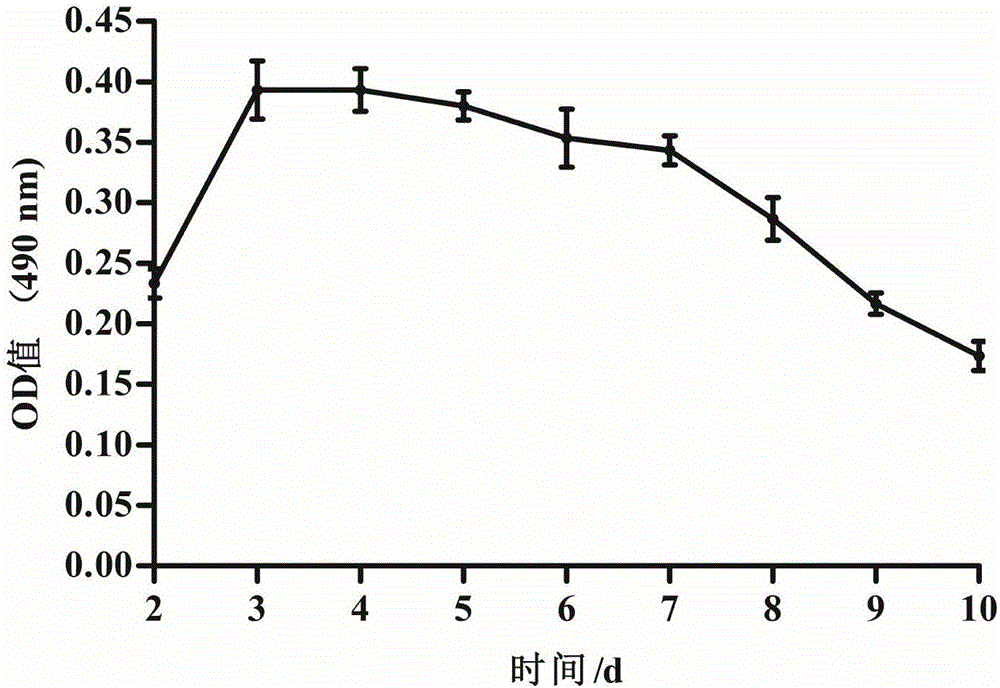
[0049] Aseptically separate tissue from the neck of the oviduct funnel near the follicle of a 26-week-old laying hen, cut the isolated tissue into pieces with a side length of 0.8 mm, and use 200 μg / mL penicillin and 200 μg / mL streptomycin. After washing the tissue with 7 mL of phosphate buffer solution to remove mucus, 0.25 wt% trypsin and 0.02 wt% EDTA were added to the tissue, and the tissue was digested in a constant temperature water bath at 37°C for 15 min. Then centrifuge at 670 rcf / min for 20 min, collect the precipitated cells, and then centrifuge the cell pellet at 670 rcf / min for 10 min. After the two centrifuges, use 10 mL of DMEM / F12 medium to suspend the precipitated tissue to obtain the suspended tissue, and use a cell sieve with a diameter of 100 μm to filter , collect the cells in the suspension tissue, centrifuge the collected cells at 670rcf / min for 10min, repeat the centrifugation twice, and then resuspend the cells with DMEM / F12 medium to obtain chicken ovi...
Embodiment 2
[0056] Aseptically isolate tissue from the neck of the oviduct funnel near the follicle of a 27-week-old laying hen, cut the isolated tissue into pieces with a side length of 0.9mm, and use penicillin 190U / mL, streptomycin 190U / mL After washing the tissue block with 5 mL of phosphate buffer solution to remove mucus, add 0.25 wt% trypsin and 0.02 wt% EDTA to the tissue, and digest the tissue in a constant temperature water bath at 37°C for 15 min. Then centrifuge at 670rcf / min for 20min, collect the precipitated cells, and then centrifuge the cell pellet at 670rcf / min for 10min.
[0057] The floating tissue was filtered with a cell sieve with a diameter of 100 μm, and the cells in the suspended tissue were collected. The collected cells were centrifuged at 670 rcf / min for 10 min, and the centrifugation was repeated twice, and then the cells were resuspended using DMEM / F12 medium to obtain chicken Fallopian tube epithelial cells. The resulting chicken oviduct epithelial cells w...
Embodiment 3
[0059] Aseptically separate tissue from the neck of the oviduct funnel near the follicle of a 25-week-old laying hen, cut the isolated tissue into pieces with a side length of 0.8mm, and use penicillin 210U / mL, streptomycin 210U / mL After washing the tissue block with 5 mL of phosphate buffer solution to remove mucus, add 0.25 wt% trypsin and 0.02 wt% EDTA to the tissue, and digest the tissue in a constant temperature water bath at 37°C for 15 min. Then centrifuge at 670 rcf / min for 20 min, collect the precipitated cells, and then centrifuge the cell pellet at 670 rcf / min for 10 min. After the two centrifuges, use 10 mL of DMEM / F12 medium to suspend the precipitated tissue to obtain the suspended tissue, and use a cell sieve with a diameter of 100 μm to filter , collect the cells in the suspension tissue, centrifuge the collected cells at 670rcf / min for 10min, repeat the centrifugation twice, and then resuspend the cells with DMEM / F12 medium to obtain chicken oviduct epithelial ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com