Completely bio-manipulated nitrophenol enhanced electrochemical degradation method
A nitrophenol, electrochemical technology, applied in the application field of microbial fuel cells, can solve problems such as increasing energy consumption, and achieve the effect of efficient removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
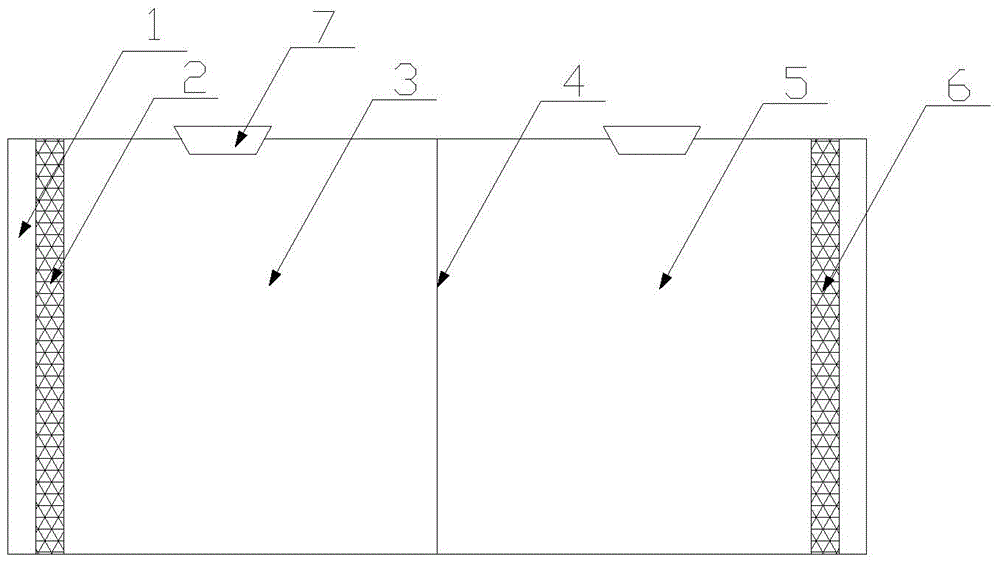
[0025] Such as figure 1 As shown, the electrode solution (containing NaH 2 PO 4 .2H 2 O5.6g / L, Na 2 HPO 4 .12H 2 O6.07g / L, NH 4 Cl310mg / L, KCl130mg / L and trace elements: FeCl 3 4H 2 O2g / L, CoCl 2 ·6H 2 O2g / L, MnCl 2 4H 2 O0.5g / L, CuCl 2 2H 2 O0.03g / L, ZnCl 2 0.05g / L, H 3 BO 3 0.05g / L, (NH 4 ) 6 Mo 7 o 24 2H 2 O0.09g / L, Na 2 SeO 3 4H 2 O0.1g / L, NiCl2 ·6H 2 (00.05g / L, EDTA 1g / L and HCl (36%w / w) 1mL / L). Sodium acetate is used as the anode carbon source and electron donor, and is added to the anode chamber 3 to make the initial COD 1000mg / L. Add 50mg / L p-nitrophenol and 10mM sodium bicarbonate into cathode chamber 5. After 50 hours of reactor operation, the degradation rate of cathode nitrophenol reached 100%, and the generation rate of aminophenol reached 48%. Afterwards, the catholyte was transferred to the anode, and after 48 hours of reactor operation, the complete degradation of aminophenol was achieved.
Embodiment 2
[0027] Such as figure 1 As shown, the electrode solution (containing NaH 2 PO 4 .2H 2 O5.6g / L, Na 2 HPO 4 .12H 2 O6.07g / L, NH 4 Cl310mg / L, KCl130mg / L and trace elements: FeCl 3 4H 2 O2g / L, CoCl 2 ·6H 2 O2g / L, MnCl 2 4H 2 O0.5g / L, CuCl 2 2H 2 O0.03g / L, ZnCl 2 0.05g / L, H 3 BO 3 0.05g / L, (NH 4 ) 6 Mo 7 o 24 2H 2 O0.09g / L, Na 2 SeO 3 4H 2 O0.1g / L, NiCl 2 ·6H 2 (00.05g / L, EDTA 1g / L and HCl (36%w / w) 1mL / L). Sodium acetate is used as the anode carbon source and electron donor, and is added to the anode chamber 3 to make the initial COD 1000mg / L. Add 100mg / L p-nitrophenol and 10mM sodium bicarbonate into cathode chamber 5. After 50 hours of reactor operation, the degradation rate of nitrophenol at the cathode reaches 73%, and the generation rate of aminophenol reaches 29%. Afterwards, the catholyte was transferred to the anode, and after 24 hours of reactor operation, the complete degradation of aminophenol was achieved.
Embodiment 3
[0029] Such as figure 1 As shown, the electrode solution (containing NaH 2 PO 4 .2H 2 O5.6g / L, Na 2 HPO 4 .12H 2 O6.07g / L, NH 4 Cl310mg / L, KCl130mg / L and trace elements: FeCl 3 4H 2 O2g / L, CoCl 2 ·6H 2 O2g / L, MnCl 2 4H 2 O0.5g / L, CuCl 2 2H 2 O0.03g / L, ZnCl 2 0.05g / L, H 3 BO 3 0.05g / L, (NH 4 ) 6 Mo 7 o 24 2H 2 O0.09g / L, Na 2 SeO 3 4H 2 O0.1g / L, NiCl 2 ·6H 2 (00.05g / L, EDTA 1g / L and HCl (36%w / w) 1mL / L). Sodium acetate is used as the anode carbon source and electron donor, and is added to the anode chamber 3 to make the initial COD 1000mg / L. Add 25 mg / L p-nitrophenol and 10 mM sodium bicarbonate into cathode chamber 5. After 45 hours of reactor operation, the degradation rate of cathode nitrophenol reached 85%, and the generation rate of aminophenol reached 34%. The catholyte was transferred to the anode chamber 3, and after 36 hours of reactor operation, the complete degradation of aminophenol was achieved.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com