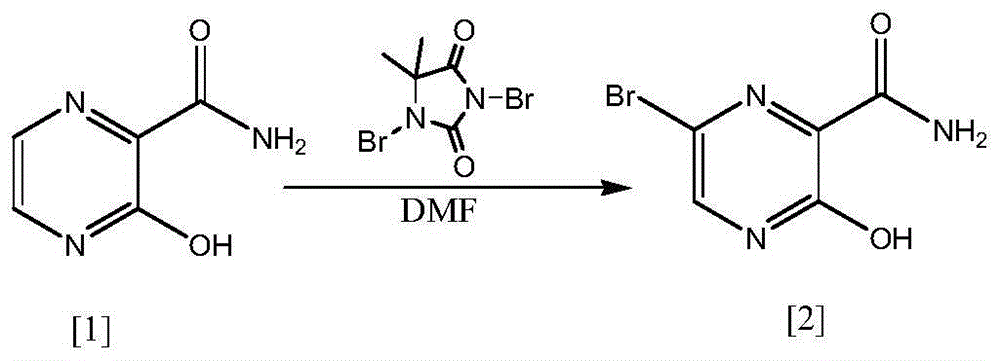
A preparing method of 6-bromo-3-hydroxy-2-pyrazinamide
A technology of pyrazinamide and hydroxyl, which is applied in the field of preparation of 6-bromo-3-hydroxy-2-pyrazinamide, can solve the problems of low total yield, difficult removal of solvent, cumbersome process, etc., and achieve convenient market procurement, The effect of a wide range of solvents and low prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Add 458g (3mol, 1eq) of methyl 3-hydroxy-2-pyrazinecarboxylate and 532g (3mol, 1eq) of N-bromosuccinimide to 5L of acetonitrile in sequence, at 25°C Stir for 12 hours, after the TLC detection reaction is complete, stop the reaction, filter, filter cake room temperature vacuum drying, obtain the product 6-bromo-3-hydroxyl-2-pyrazinecarboxylic acid methyl ester of 554g (comprising its tautomer 6 -Bromo-3-oxo-3,4-dihydro-2-pyrazinecarboxylate) with a yield of 85%. 6-Bromo-3-hydroxyl-2-pyrazinecarboxylate Methyl NMR and mass spectrometry indicators are as follows:
[0021] 1 H-NMR (CDCl 3 ,600MHz) δ value: 4.09 (3H,s,CH 3 ),8.53(1H,s,pyrazineH)
[0022] MS(ESI)m / z:233.1[M+H] + ,235.2[M+2+H] +
[0023] (2) Dissolve 21g (0.14mol) of methyl 6-bromo-3-hydroxy-2-pyrazinecarboxylate in 450mL of tetrahydrofuran, add 257ml of concentrated ammonia water (mass fraction 25%) under stirring, at 25°C After 7 hours of reaction, TLC detected that the reaction was complete, and...
Embodiment 2
[0027] (1) Add 308g (2mol, 1eq) of methyl 3-hydroxy-2-pyrazinecarboxylate and 956g (6mol, 3eq) of liquid bromine into 4L of acetonitrile, stir at 27°C for one hour, and TLC detects that the reaction is complete , the reaction solution was poured into 4L of water, the excess liquid bromine was removed with a saturated aqueous solution of sodium sulfite, left to filter, and the filter cake was vacuum-dried at room temperature to obtain 300 g of the product 6-bromo-3-hydroxy-2-pyrazinecarboxylic acid methyl Esters (including its tautomer 6-bromo-3-oxo-3,4-dihydro-2-pyrazinecarboxylate methyl ester) in a yield of 65%. 6-Bromo-3-hydroxyl-2-pyrazinecarboxylate Methyl NMR and mass spectrometry indicators are as follows:
[0028] 1 H-NMR (CDCl 3 ,600MHz) δ value: 4.09 (3H,s,CH 3 ),8.53(1H,s,pyrazineH),11.45(1H,s,OH)
[0029] MS(ESI)m / z:233.1[M+H] + ,235.2[M+2H] (2+) ,255.1[M+Na] +
[0030] (2) Dissolve 10g (0.04mol) of methyl 6-bromo-3-hydroxy-2-pyrazinecarboxylate in 200mL of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com