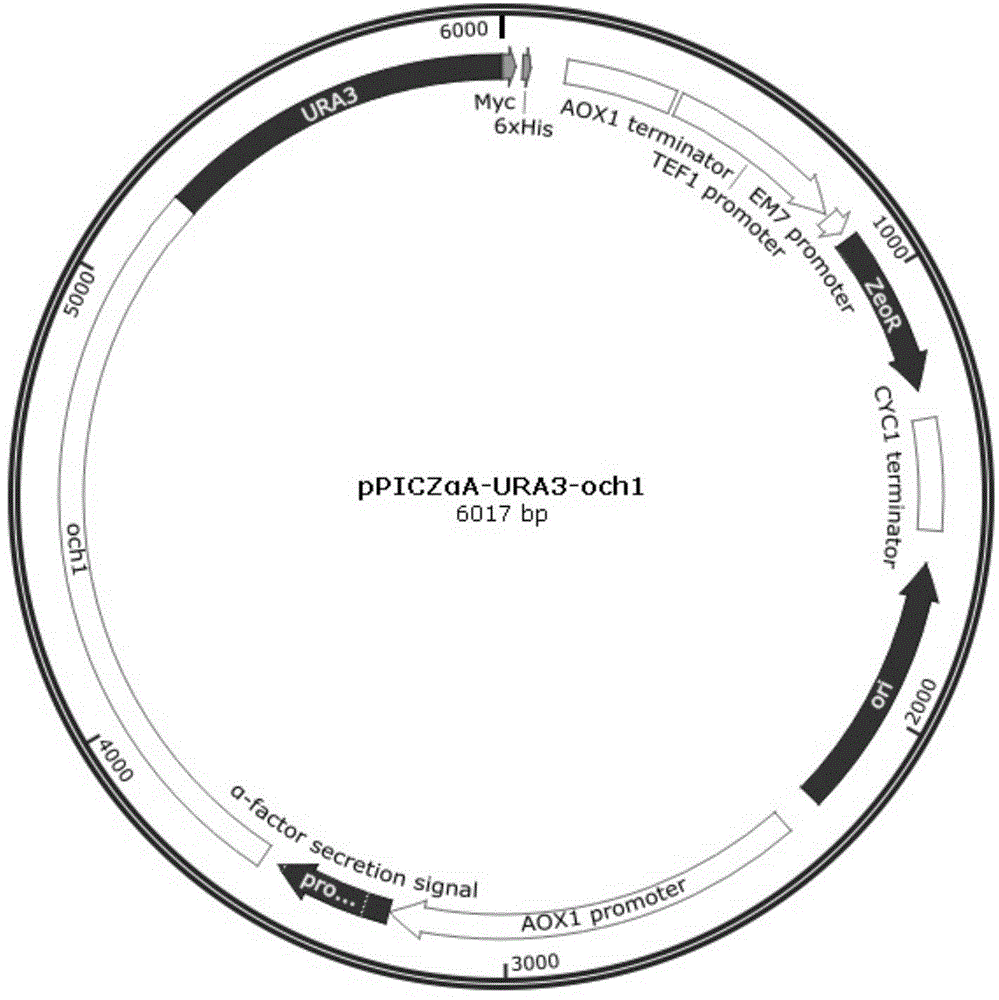
Construction method of Pichia pastoris expressed by OCH1 defect anti-CD20 tetravalent antibody
A Pichia pastoris and construction method technology, applied in the field of genetic engineering, can solve problems such as inconvenient production of pharmaceutical proteins, lack of eukaryotic protein modification and processing systems, and incorrect spatial conformation of endogenous protease degradation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0031] A method for constructing an OCH1-deficient anti-CD20 tetravalent antibody expressing Pichia pastoris, comprising the following steps:
[0032] 1. Construction of positive clone Δoch1 strain
[0033] 1) Construct fusion gene ΔOCH1
[0034] Two pairs of nucleotide primers OCH1-5F, OCH1-5R, OCH1-3F, OCH1-3R were designed according to the Pichia pastoris OCH1 gene sequence reported in Genebank (E12456). Using the genome of the X-33 strain as a template, the homology arm sequences at both ends of OCH1 were cloned. OCH1-5F, the product of OCH1-5R clone SEQ ID NO: 10; OCH1-3F, the sequence of the product of OCH1-3R clone is as SEQ ID NO: 11
[0035] Figure 4 : OCH1 left arm (5' end) and right arm (3' end) clone electrophoresis pattern, the reading frame sequence of about 1,200 bp is missing between the homologous arms on both sides, the size of the fusion fragment och1 is about 1600 bp, and the sequence is as SEQ ID NO: 12, so The open reading frame of the OCH1 gene was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com