Chlorotoxin conjugates and methods of use thereof
A compound and alkylene technology, applied in the field of chlorotoxin conjugates and its use, can solve the problems of dependence, inaccurate intraoperative identification, and limitation of the degree of surgical resection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0839] The invention is further illustrated by the following non-limiting examples.
example 1
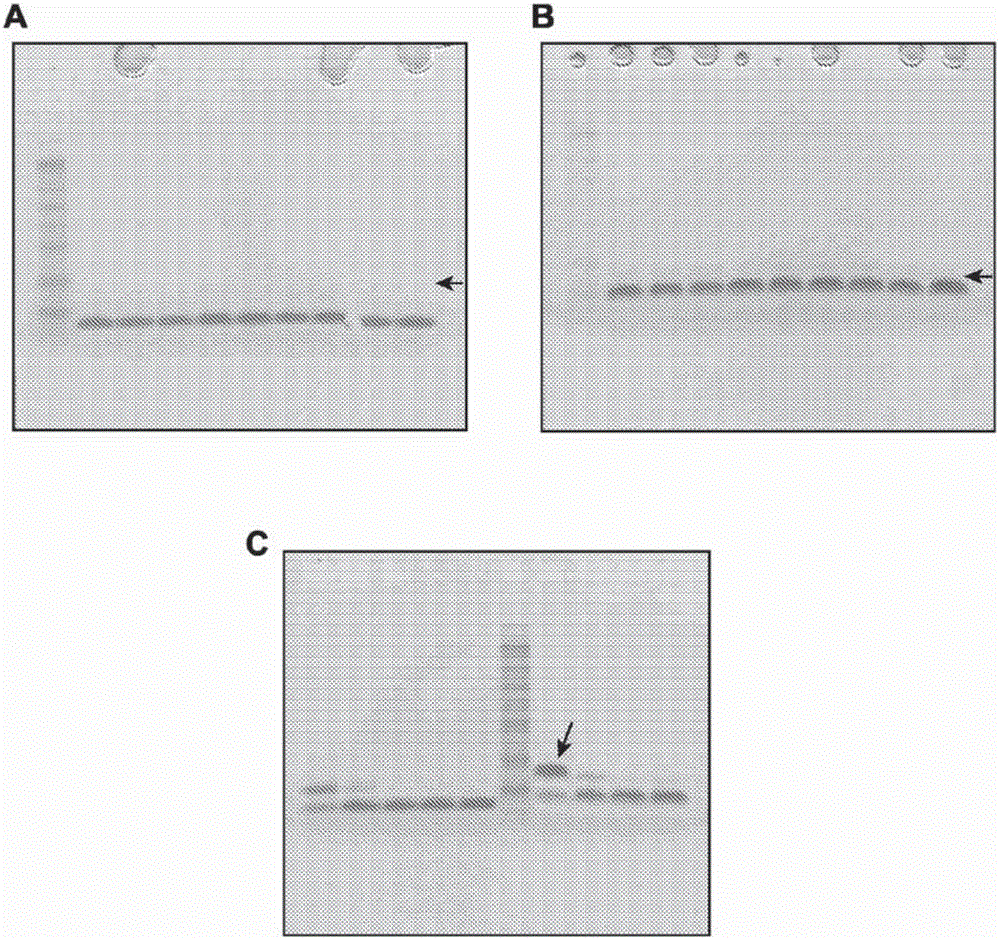
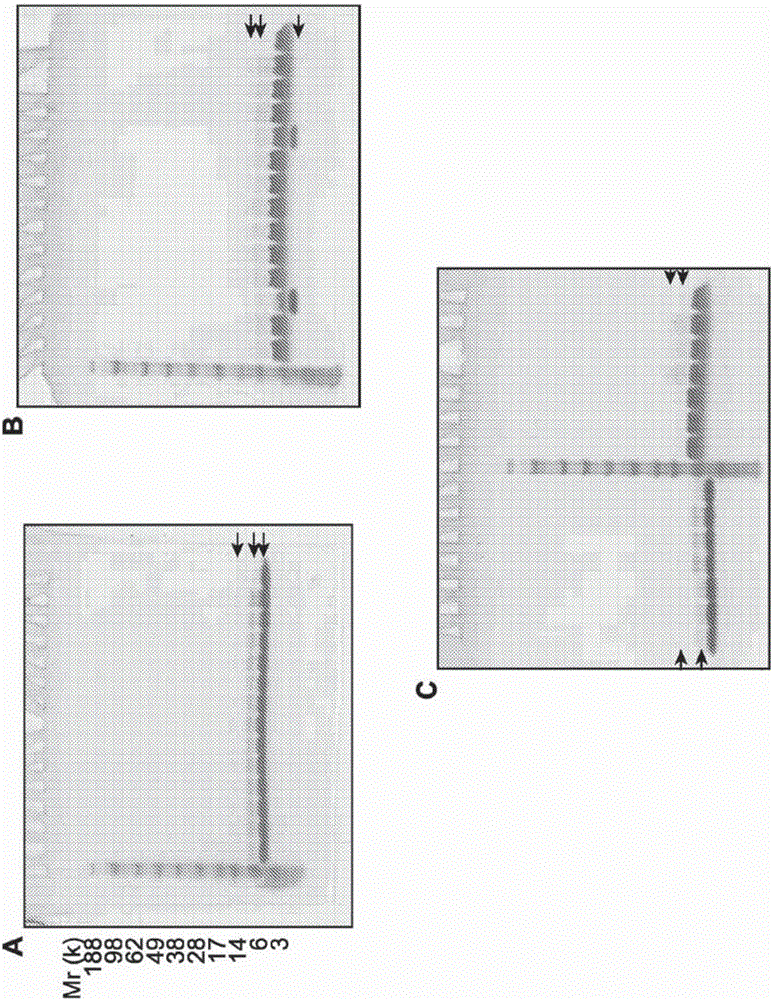
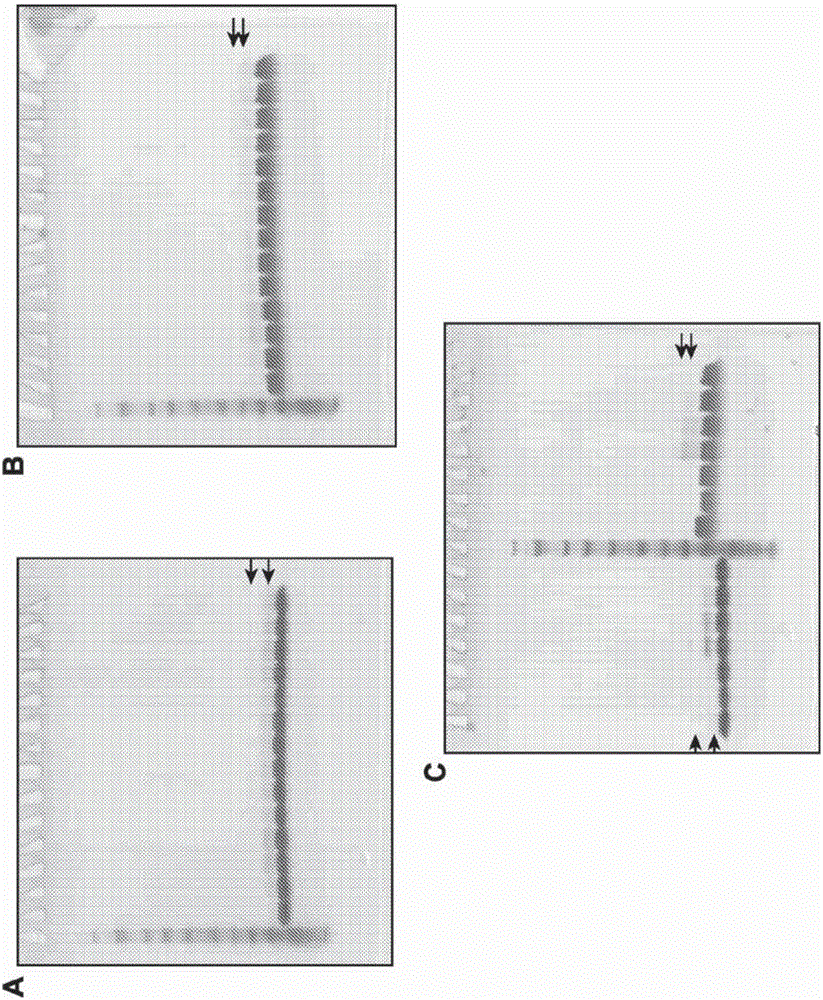
[0841] Stability of compound 16 with ammonium acetate salt
[0842]
[0843] A = MCMPCFTTDHQMARRCDDCCGGRGRGKCYGPQCLCR (K-27 is the attachment point)
[0844] This example demonstrates the production and stability of compound 16 over time under storage conditions at different pH and temperature.
[0845] The peptide portion of compound 16 is a targeting peptide (modified chlorotoxin) conjugated to a fluorescent dye. The targeting peptide selectively binds to cancerous cells and the dye moiety facilitates detection by imaging. The peptide is a modified chlorotoxin of 36 amino acids (with H-Met-Cys-Met-Pro-Cys-Phe-Thr-Thr-Asp-His-Gln-Met-Ala-Arg-Arg-Cys-Asp-Asp - the sequence of Cys-Cys-Gly-Gly-Arg-Gly-Arg-Gly-Lys-Cys-Tyr-Gly-Pro-Gln-Cys-Leu-Cys-Arg-OH) wherein three lysines in the natural chlorotoxin Two of the acidic amino acids were replaced by arginines (K15R and K23R) to facilitate subsequent conjugation of the fluorophore to a single residual lysine (K27) residue, thu...
example 2
[0872] Evaluation of the stability of compound 16 in different buffers and at different pH
[0873]
[0874] A = MCMPCFTTDHQMARRCDDCCGGRGRGKCYGPQCLCR (K-27 is the attachment point)
[0875] This example shows the stability of compound 16 with respect to pH and provides an evaluation using alternative buffers and different excipients and formulations.
[0876] This example further shows that different types of excipients protect compound 16 from heat, light, oxidation and freeze / thaw stress.
[0877] In some cases, the formulations were stored in microcentrifuge tubes in an air atmosphere and after 2d and 5d at 40°C in the dark, in the dark (dk) or at room temperature (rt) in ambient light (lt) Assays were performed after 3 d, and after 3X freeze-thaw (F / T) between -20°C and room temperature in the dark. By visual inspection, centrifugation, RP-HPLC, A 786 Formulations were evaluated by concentration, pH and SDS-PAGE.
[0878] In some cases, the time points were 4, 7, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| emission peak | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com