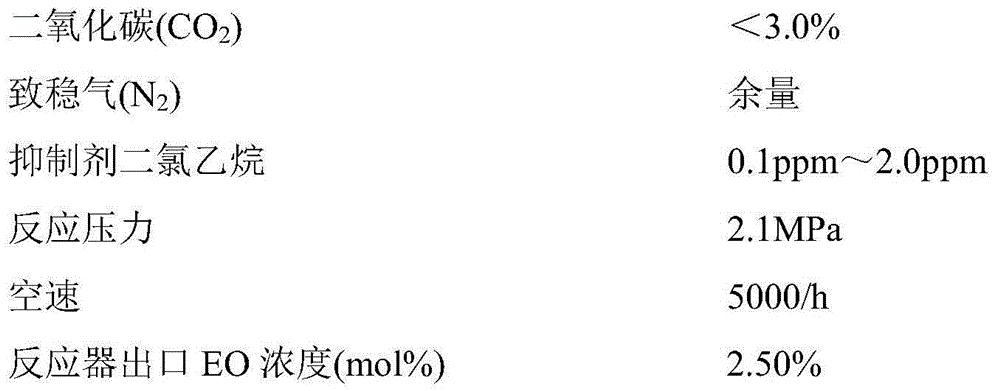Method for preparing silver catalyst, silver catalyst and application thereof of silver catalyst
A technology of silver catalyst and auxiliary agent, applied in the field of preparation of silver catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Dissolve 0.034g of methyl rhenium trioxide in 50g of deionized water to prepare an organic rhenium-containing aqueous solution with a rhenium concentration of 500ppm. Take another 15g carrier sample and soak it in the solution prepared above for 30min at room temperature, filter the solution and place the carrier in an oven. Dry at 120°C for 30 minutes to obtain the treated carrier; then dissolve 8.70g of ethylenediamine and 2.94g of ethanolamine in 18.06g of deionized water, and slowly add silver oxalate into the mixture under stirring. The amount of silver oxalate added makes the final preparation Contain silver 26% by weight in the impregnating liquid that obtains, then add the cesium sulfate of 0.068g and be mixed with impregnating liquid soaking the treated carrier, then make silver catalyst finished product after leaching and 300 ℃, thermal decomposition in 3 minutes, its performance As shown in Table 1.
Embodiment 2
[0071] Dissolve 0.034g of methyl rhenium trioxide in 50g of deionized water to prepare an organic rhenium-containing aqueous solution with a rhenium concentration of 500ppm. Another 15g carrier sample is put into a flask and soaked in the above prepared solution for 30min at room temperature, and the solution is filtered off. Dry the carrier in an oven at 80°C for 120 minutes to obtain the treated carrier; then dissolve 8.70g of ethylenediamine and 2.94g of ethanolamine in 18.06g of deionized water, and slowly add silver oxalate to the mixture under stirring. The addition of silver oxalate Quantity makes in the impregnating liquid that finally makes contains silver 18% by weight, then adds the cesium sulfate of 0.068g and is mixed with impregnating liquid and soaks the treated carrier, makes silver catalyst after leaching and 300 ℃, thermal decomposition in 3 minutes The properties of the finished product are shown in Table 1.
Embodiment 3
[0073] Dissolve 0.034g of methyl rhenium trioxide in 50g of deionized water to prepare an organic rhenium-containing aqueous solution with a rhenium concentration of 500ppm. Another 15g carrier sample is put into a flask and soaked in the above prepared solution for 30min at room temperature, and the solution is filtered off. Dry the carrier in an oven at 90°C for 60 minutes to obtain the treated carrier; then dissolve 8.70g of ethylenediamine and 2.94g of ethanolamine in 18.06g of deionized water, and slowly add silver oxalate to the mixture under stirring. The addition of silver oxalate Quantity makes in the impregnating liquid that finally makes contains silver 37% by weight, then adds the cesium sulfate of 0.068g and is mixed with impregnating liquid and soaks the treated carrier, makes silver catalyst after leaching and 300 ℃, 3 minutes thermal decomposition The properties of the finished product are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com