Scandate green phosphor and preparation method thereof
A technology of green fluorescent powder and scandate, applied in chemical instruments and methods, luminescent materials, electrical components, etc., can solve the problems of short life, easy aging of packaging materials, and difficult preparation of high-efficiency power UVLEDs, etc., to improve color rendering sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The present invention also provides a method for preparing the above-mentioned fluorescent powder, comprising: mixing Sr precursor, Ce precursor, D precursor, Ca precursor and Sc precursor, performing a high-temperature solid-state reaction to obtain the fluorescent powder;
[0053] The molar ratio of Sr, Ce, D, Ca and Sc in the Sr precursor, Ce precursor, D precursor, Ca precursor and Sc precursor is (1-2x): x: x: 2: 6; 0 <x<0.5; the D is at least one of Li and Na.
[0054] Wherein, the x and D are the same as those described above, and will not be repeated here.
[0055] The Sr precursor can be a compound containing Sr well known in the art, and there is no special limitation. In the present invention, it is preferably a carbonate of Sr, an oxide of Sr, an oxalate of Sr, a nitrate of Sr, etc. At least one of, more preferably carbonate of Sr; the Ce precursor is at least one of carbonate of Ce, oxide of Ce, oxalate of Ce and nitrate of Ce, etc., more It is preferably...
Embodiment 1
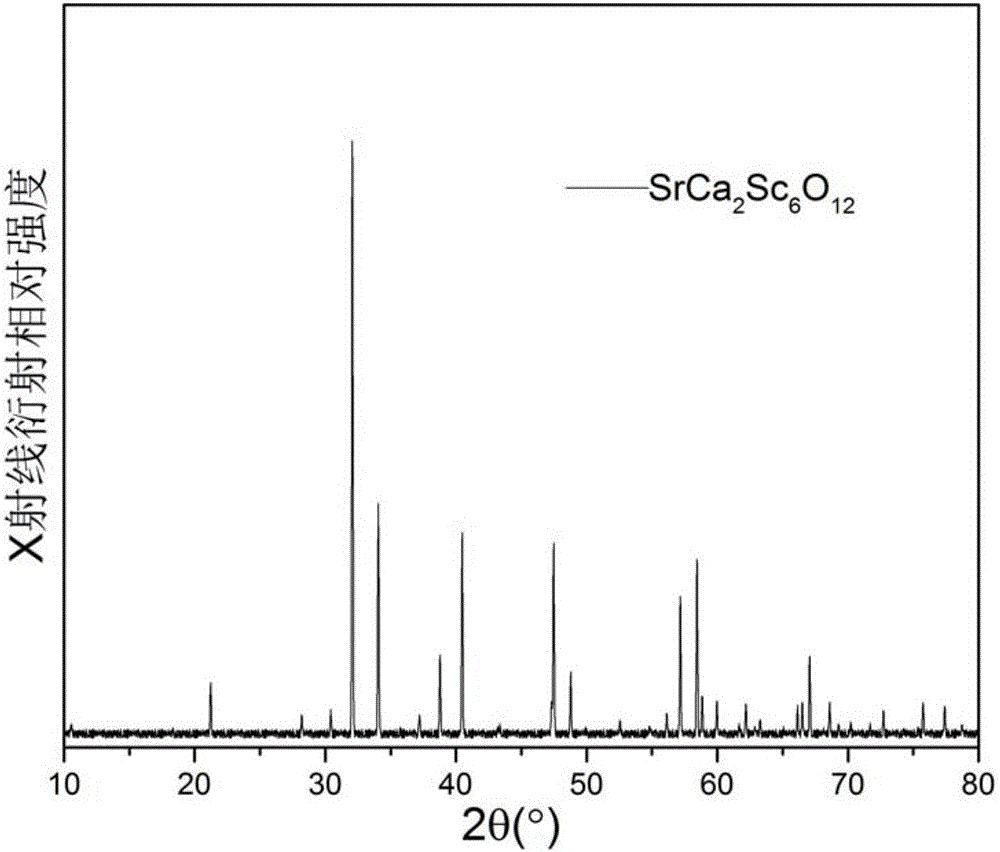
[0065] The raw material is SrCO 3 (analytical pure), CaCO 3 (analytically pure) and Sc 2 o 3 (Analytical pure), the molar ratio is 1:2:3, the above raw materials are ground and mixed, dried, pressed into tablets under a pressure of 2 MPa, put into a crucible, sintered in a high-temperature furnace at 1350 ° C for 10 h, and cooled to room temperature with the furnace , the theoretical chemical composition is obtained as SrCa 2 sc 6 o 12 s material.
[0066] Utilize X-ray diffraction to analyze the material obtained in embodiment 1, obtain its X-ray diffraction pattern, as figure 1 shown. Inquire in the international crystallographic database and confirm that this spectrum belongs to the hexagonal space group as P6 3 / m and the chemical composition is SrCa 2 sc 6 o 12 The standard spectrum is consistent.
Embodiment 2
[0083] The raw material is SrCO 3 (analytical pure), CeO 2 (99.99%), Li 2 CO 3 (analytical pure), CaCO 3 (analytically pure) and Sc 2 o 3 (Analytical pure), the molar ratio is 0.98:0.01:0.01:2:3, the above raw materials are ground and mixed, dried and pressed into tablets under a pressure of 1 MPa, put into a crucible, and under an ammonia reducing atmosphere, in a high-temperature furnace, Sintering at 1350°C for 10h, cooling to room temperature with the furnace, the theoretical chemical composition is Sr 0.98 Ce 0.01 Li 0.01 Ca 2 sc 6 o 12 of fluorescent powder.
[0084] Utilize fluorescence spectrometer to analyze the fluorescent material obtained in embodiment 2, obtain its excitation spectrogram, as Figure 8 shown. It can be seen that the excitation bands of the phosphor are distributed in both the violet-blue light region and the ultraviolet light region.
[0085] Utilize fluorescence spectrometer to analyze the fluorescent material obtained in embodiment ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com