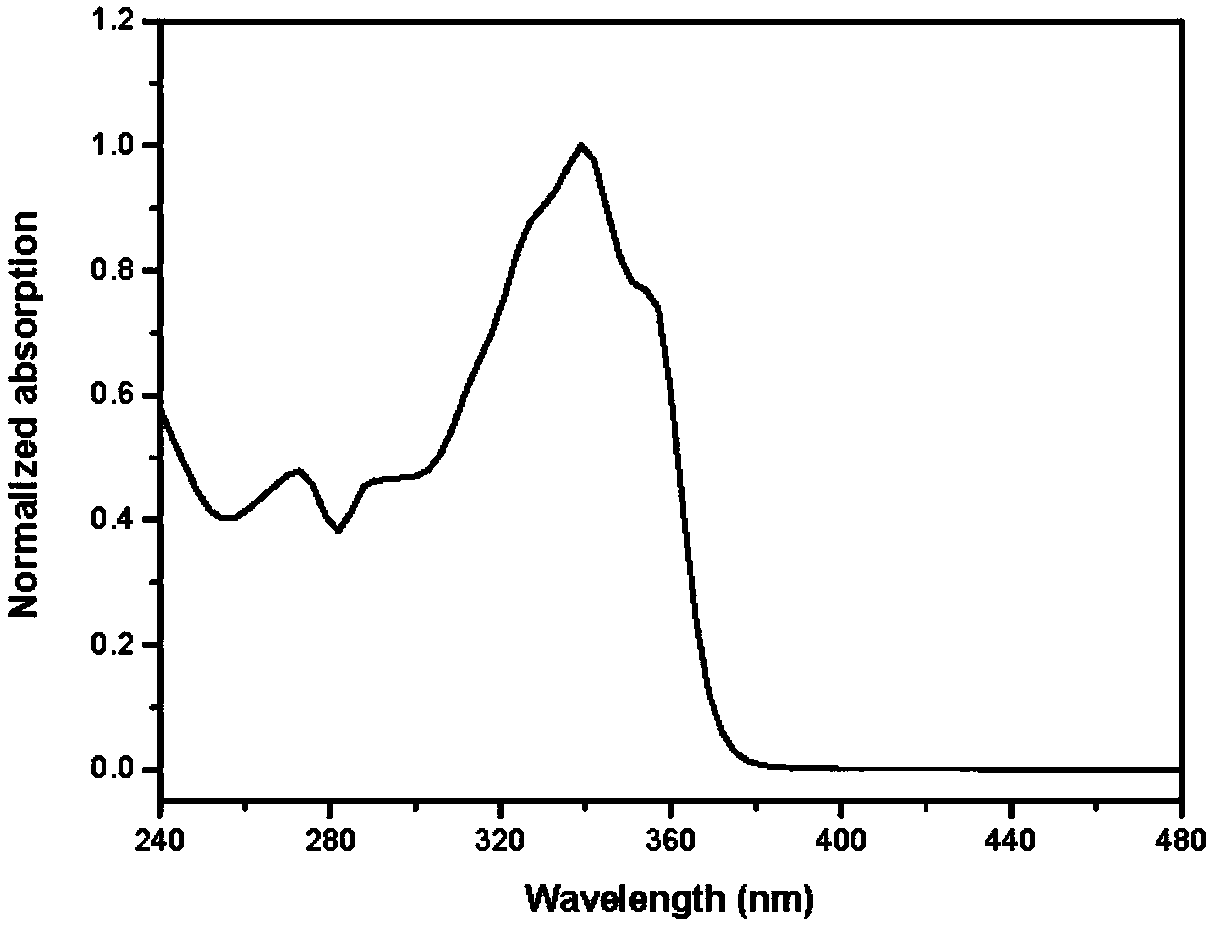
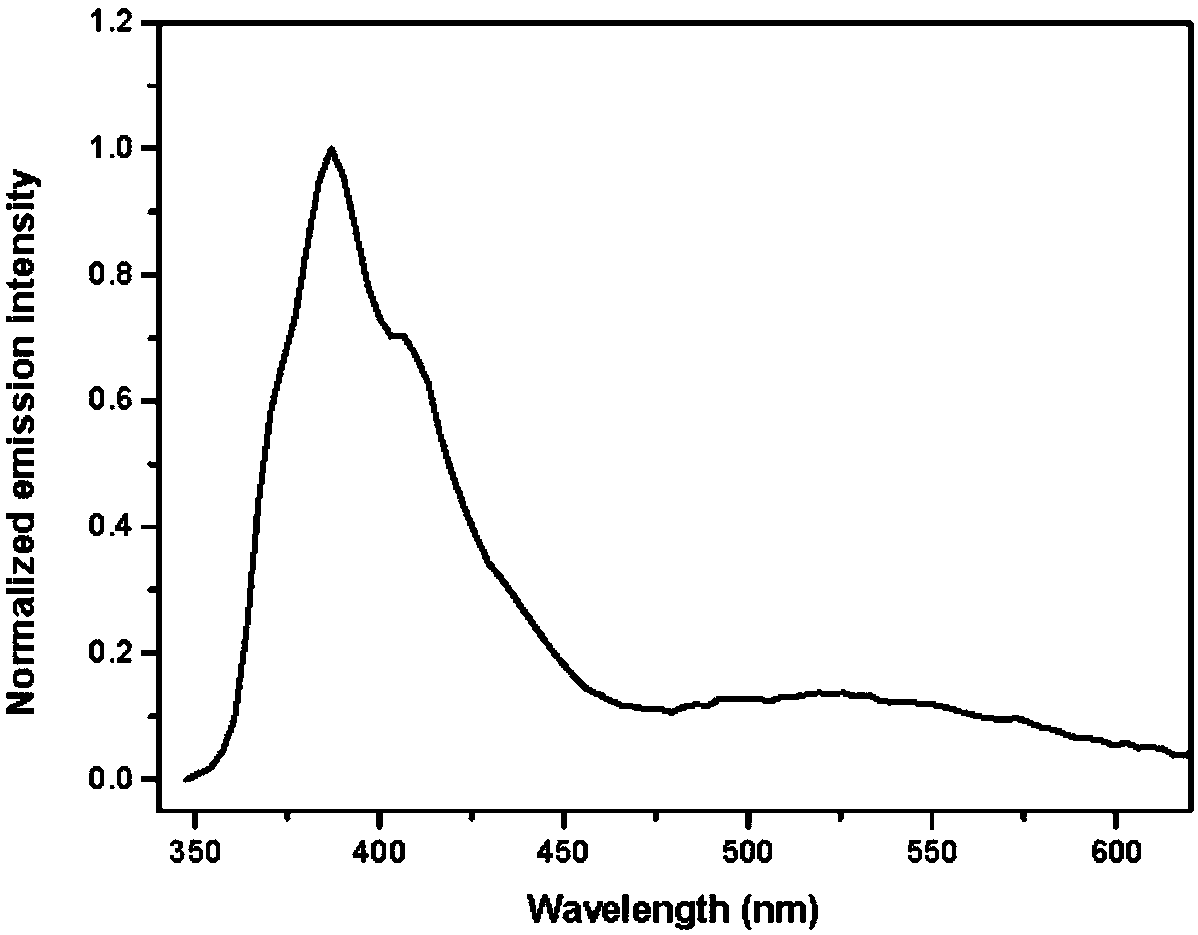
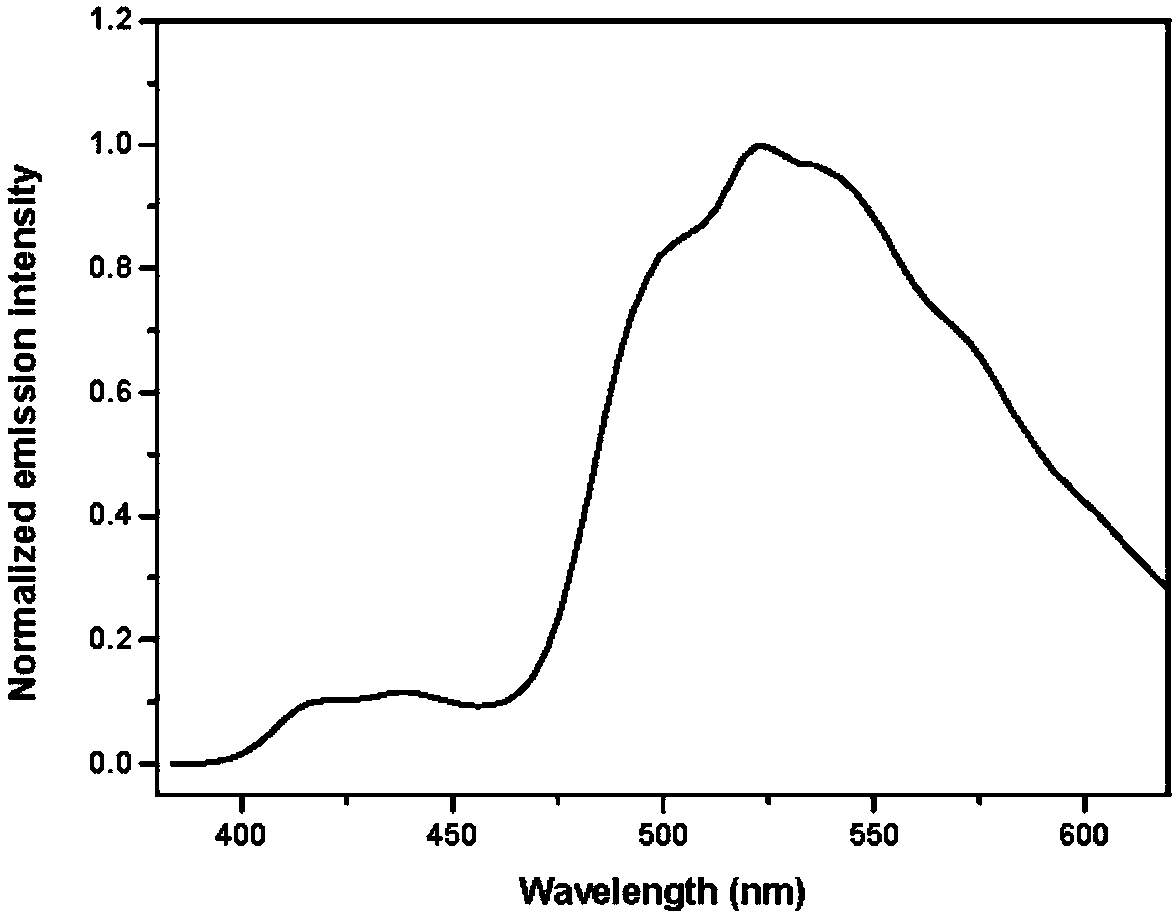
A fluorenyl β-carboline compound, its application and preparation method as an organic light-emitting material and an aggregation-induced fluorescence enhancement material
A technology for aggregation-induced fluorescence and light-emitting materials, applied in the field of organic light-emitting materials, can solve the problems of few types of compounds, poor structural modifiability, complex device preparation, etc., and achieves the effects of good film formation, few synthesis steps, and easy purification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Preparation of N'-(2-fluorenylidene)-β-carboline-3-formylhydrazide (I)
[0053] (1) Preparation of β-carboline-3-formylhydrazide (compound III)
[0054] In a 100 ml round bottom flask, dissolve ethyl β-carboline-3-carboxylate (compound II) and hydrazine hydrate (80%) at a weight ratio of 1:5 in 50 ml of methanol, and reflux under rapid stirring After 7 hours, after the reaction was completed, it was cooled to room temperature, and the obtained solid matter was suction-filtered under reduced pressure, washed with water three times, and dried at room temperature. Recrystallized from ethanol and dried in vacuo to obtain β-carboline-3-carboxylhydrazide (compound III) in the form of silvery white flakes, with a yield of 82%, ESI-MS m / z: 227.3 (M+H) + ;
[0055] (2) Preparation of N'-(2-fluorenylidene)-β-carboline-3-formylhydrazide (compound I)
[0056] In a dry 100 ml round bottom flask, add intermediate β-carboline-3-formylhydrazide (compound III) (0.002mol), ...
Embodiment 2
[0058] Example 2: Preparation of N'-(2-fluorenylidene)-β-carboline-3-formylhydrazide (I)
[0059] According to the method of embodiment 1, obtain by two-step reaction, difference is, in step (1), the weight ratio of β-carboline-3-formic acid ethyl ester and hydrazine hydrate (80%) is 1:9, reaction The organic solvent used is ethanol, the reaction time is 9 hours, and the productive rate is 84%; The reaction was stirred and refluxed for 8 hours, and the mixed solvent used for recrystallization was ethanol-acetone. Yield 87%.
Embodiment 3
[0060] Example 3: Preparation of N'-(2-fluorenylidene)-β-carboline-3-formylhydrazide (I)
[0061] According to the method of embodiment 1, obtain by two-step reaction, difference is, in step (1), the weight ratio of β-carboline-3-formic acid ethyl ester and hydrazine hydrate (80%) is 1:7, reaction The organic solvent used is ethanol, the reaction time is 8 hours, and the productive rate is 83%; The reaction was stirred and refluxed for 7 hours, and the mixed solvent used for recrystallization was ethanol-acetone. Yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com