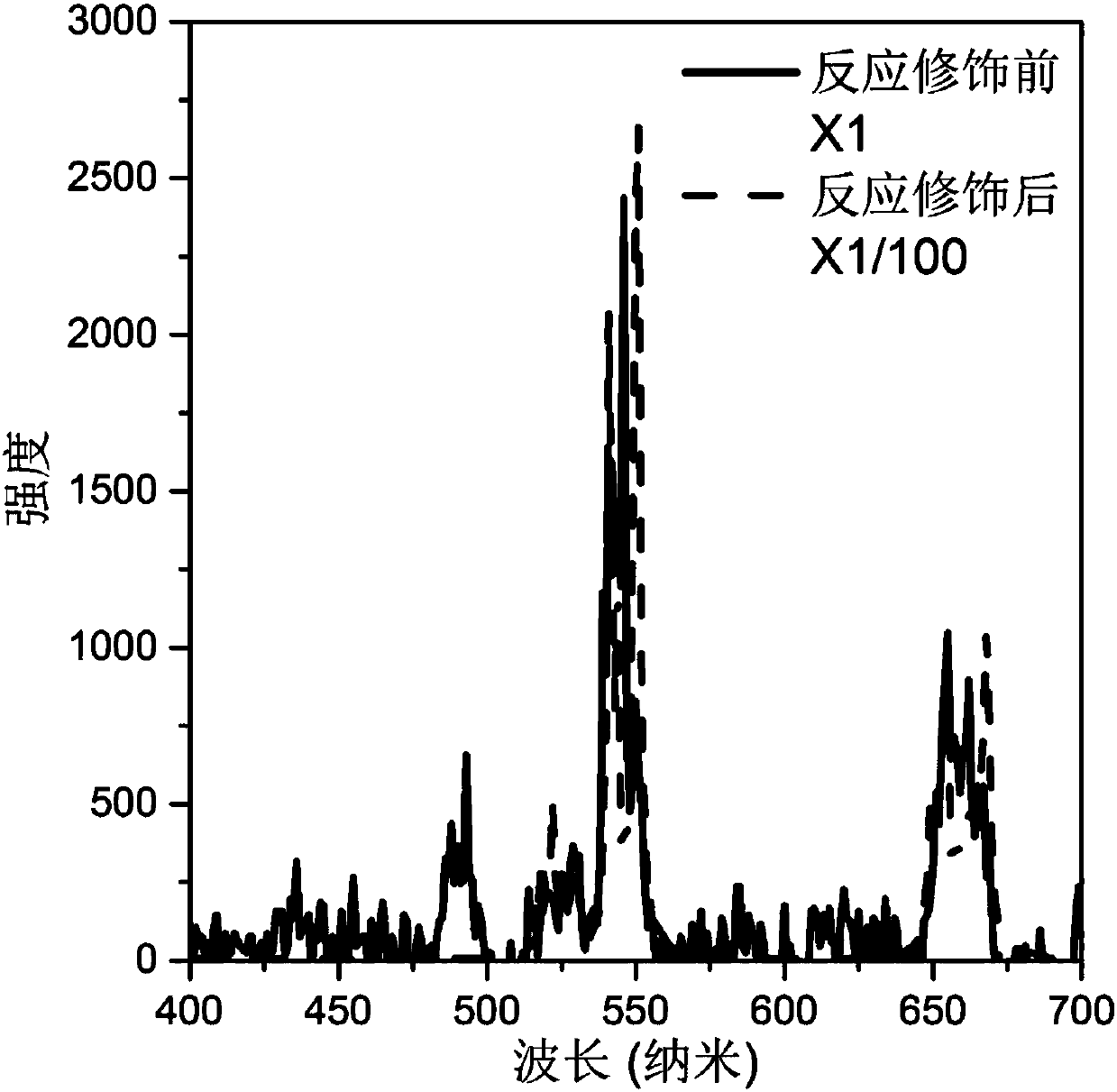
Method for Improving Rare Earth Ion Doped Inorganic Fluoride Upconversion Luminescence Intensity
A rare earth ion, luminous intensity technology, applied in chemical instruments and methods, luminescent materials, etc., can solve the problems of high cost and complicated operation, and achieve the effects of low cost, reduced defect content, and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 of the inventive method comprises the following steps:
[0024] (1) Using sodium citrate as rare earth ion complexing agent to hydrothermally synthesize β-NaYF 4 :20mol%Yb 3+ ,2mol%Er 3+ Fluoride powder crystals. Measure 2.6ml of Y(NO 3 ) 3 solution, 2.0ml concentration of 0.1M Yb(NO 3 ) 3 Solution, 0.4ml concentration 0.05M Er(NO 3 ) 3 Solution, 10ml of aqueous solution, 0.5882g of sodium citrate dihydrate were mixed and stirred for 30min. Then add 15 ml of NaF aqueous solution containing 0.5249 g to this solution, and mix and stir for 15 min. Finally, the mixed solution was transferred to a 50ml polytetrafluoroethylene bottle, put into a stainless steel autoclave, sealed, heated to 180°C, and kept warm for 24h. Naturally cooled to room temperature, the synthesized crystals were obtained by centrifugation, with a size of about 2.5 μm and a hexagonal disk shape. Dry it in an oven at 80°C for 12 hours before use. Weigh 0.0902 g of the synthesized...
Embodiment 2
[0029] Embodiment 2 of the inventive method comprises the following steps:
[0030] (1) Select the β-NaYF synthesized in Example 1 for use 4 :20mol%Yb 3+ ,2mol%Er 3+ Fluoride powder crystal is used as the object to be modified, weighing 0.4806g. Select NaF and NH 4 HF 2 As the carrier of alkali metal cations and fluoride ions, weigh NaF0.1105g, NH 4 HF 2 0.2744g.
[0031] (2) Measure 4.0ml of H 2 O, added to the solid mixture, mixed and stirred for 5min.
[0032] (3) The mixed system was transferred to a 25ml polytetrafluoroethylene bottle, put into a stainless steel autoclave, sealed, heated to 200°C, and reacted for 24h.
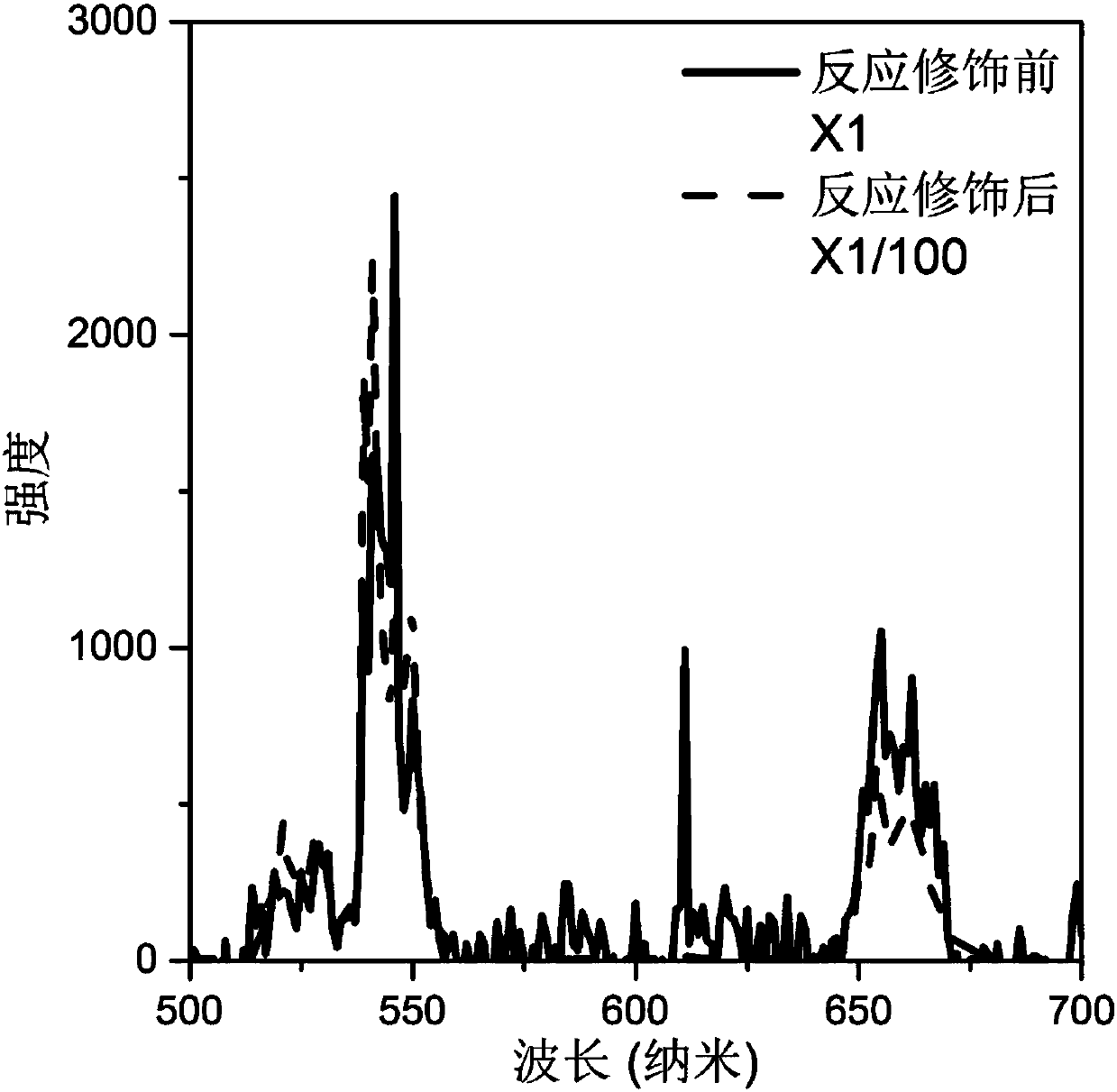
[0033] (4) After the reaction, cool naturally to room temperature, perform centrifugal washing with water, and dry at 60° C. to obtain a modified rare earth ion-doped inorganic fluoride. The luminescence spectra of the synthesized crystals under 980nm laser excitation before and after the modification reaction are in figure 2 displayed in .
Embodiment 3
[0035] Embodiment 3 of the inventive method comprises the following steps:
[0036] (1) Select the β-NaYF synthesized in Example 1 for use 4 :20mol%Yb 3+ ,2mol%Er 3+ Fluoride powder crystal is used as the object to be modified, weighing 0.1605g. LiF was selected as the alkali metal cation and fluoride ion carrier, weighing 0.1541g.
[0037] (2) Measure 1.6ml of H 2 O, added to the solid mixture, mixed and stirred for 5min.
[0038] (3) The mixed system was transferred to a 25ml polytetrafluoroethylene bottle, put into a stainless steel autoclave, sealed, heated to 220°C, and reacted for 16h.
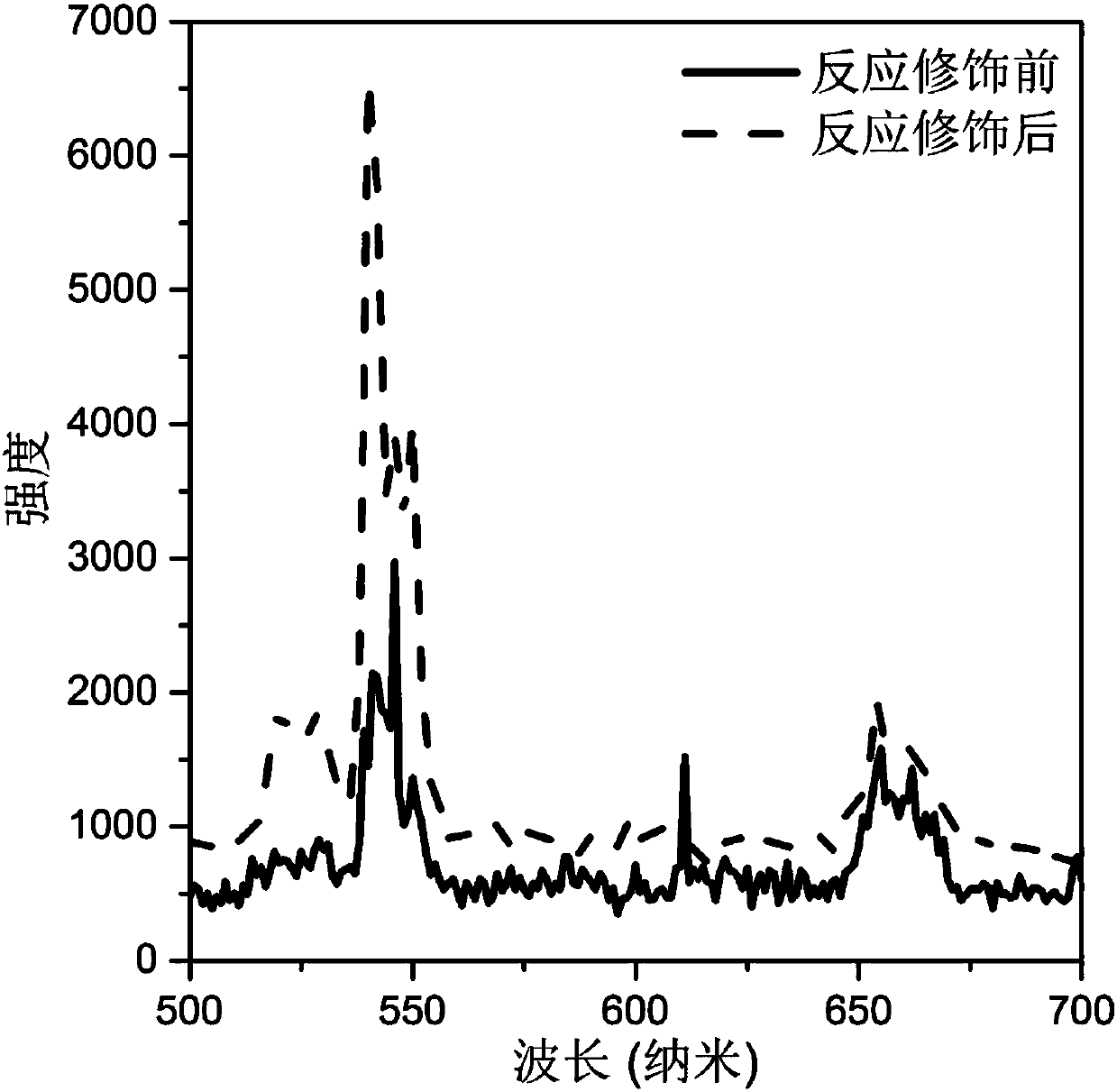
[0039] (4) After the reaction, cool naturally to room temperature, perform centrifugal washing with water, and dry at 60° C. to obtain a modified rare earth ion-doped inorganic fluoride. The luminescence spectra of the synthesized crystals under 980nm laser excitation before and after the modification reaction are in image 3 displayed in .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com