Application of baicalein to preparation of medicament for preventing and treating Parkinson's disease
A technology of baicalein and medicine, which is applied in the field of preparation of pharmaceutical preparations for the prevention and treatment of Parkinson's disease, can solve the problems of disease exacerbation, toxic side effects, and inability to delay the disease process, and achieve the goal of promoting reversal or recovery, inhibiting development, and protecting pathological changes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
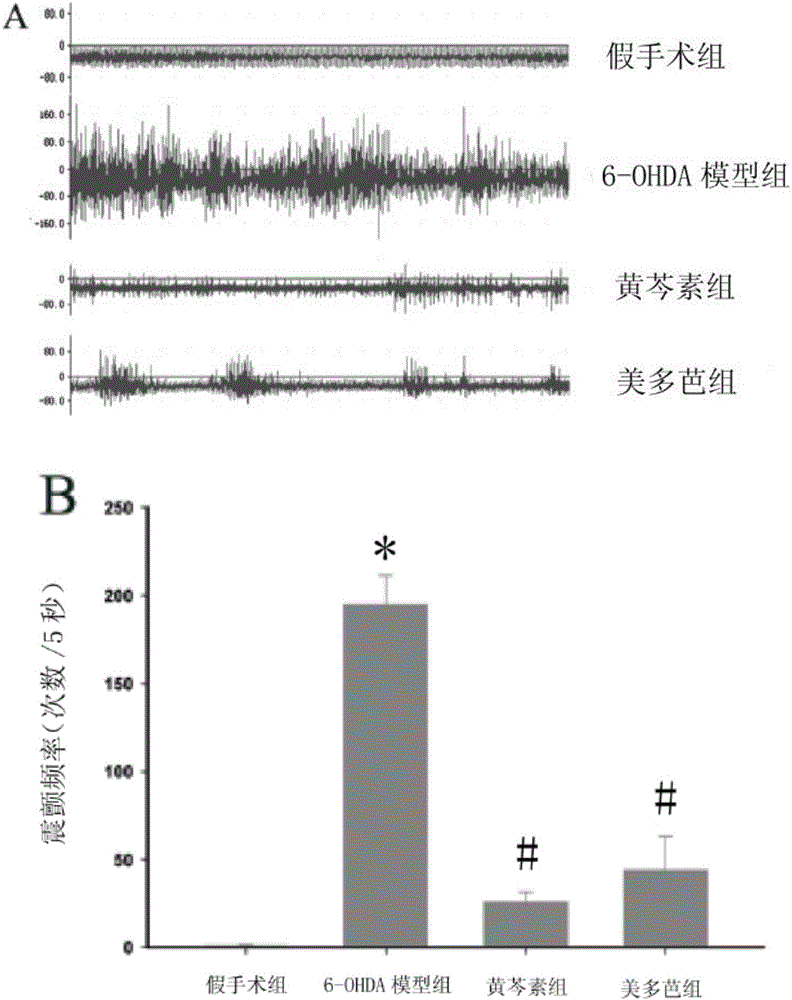
[0069] Embodiment 1, the effect of baicalein on the behavioral improvement of MPTP-induced PD mice
[0070] Model building and grouping
[0071] C57BL / 6 mice were randomly divided into 3 groups, namely normal control group, MPTP model group and baicalein administration group (200mg / kg), 12 in each group. The control group and the model group were pre-administered with normal saline, and the administration group was given the above-mentioned dose of drugs. After continuous intragastric administration for one week, the model group and the administration group were intraperitoneally injected with MPTP (30 mg / kg) on the 8th day, once a day , for 5 consecutive days, and observe the behavioral indicators of the mice on the 13th day.
[0072] Behavioral testing
[0073] 1. Spontaneous activity experiment uses mouse autonomic activity instrument (ZIL-2 type mouse autonomic activity instrument, Institute of Materia Medica, Chinese Academy of Medical Sciences) to measure and count t...
Embodiment 2
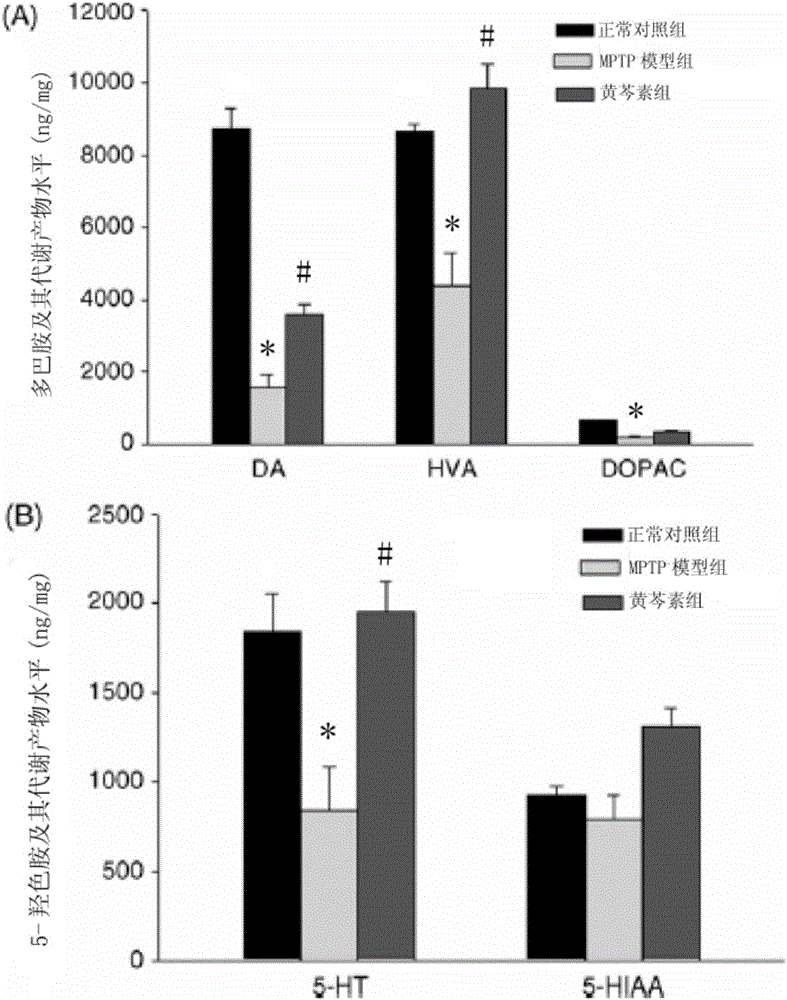
[0082] Example 2, the effect of baicalein on monoamine transmitters in the brain striatum of MPTP-induced PD mice
[0083] Model building and grouping are the same as above. Six mice were collected from each group, the brains were decapitated, and the substantia nigra and striatum were separated on ice trays and stored in liquid nitrogen. One side of the striatum was taken for the determination of neurotransmitters. Take striatum, weigh and record, homogenize in 0.2ml homogenate solution in ice bath for 40s, 80% ultrasonic energy, cycle=0.7, centrifuge at 12000rpm at 4°C for 30min, take the supernatant and detect it with high performance liquid phase electrochemical method The levels of dopamine (DA) and its metabolite dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxytryptamine (5-HT) and its metabolite 5-hydroxyindoleacetic acid (5-HIAA), each Inject 20 μl each time. Mobile phase: 0.1mol / l NaH 2 PO 4 The aqueous solution contains 0.85mmol / l OSA, 0.5m...
Embodiment 3
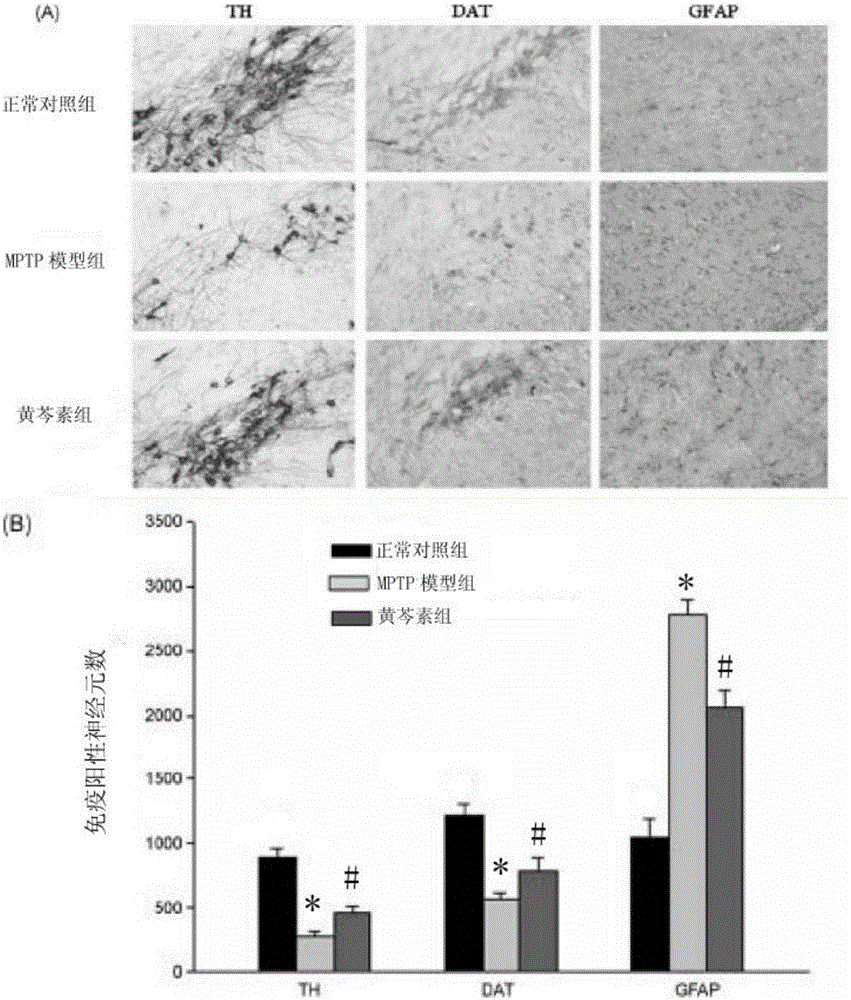
[0085] Embodiment 3, the effect of baicalein on MPTP-induced PD mouse brain tyrosine hydroxylase (TH), dopamine transporter (DAT), glial-derived fibrin (GFAP) expression
[0086] Model building and grouping are the same as above. Six rats were taken from each group for immunohistochemical detection. Anesthetize and fix with 4% chloral hydrate, open the chest cavity, perfuse and fix with normal saline and 4% paraformaldehyde respectively, and take out the brain by craniotomy. After being fixed in 4% paraformaldehyde for 1 day, the cells were replaced with 0.01M PBS containing 30% sucrose. After the brain tissue sank, a 20 μm thick coronal slice of the brain was cut with a cryostat. The brain slices were shaken and rinsed 3 times with PBST (PH=7.4), 10 min each time; 2 o 2 Treat for 10 minutes, wash with PBST shaking 3 times, 10 minutes each time; incubate with blocking solution for 1 hour; add primary antibody (TH, 1:500; DAT, 1:200; GFAP, 1:400), and incubate overnight at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com