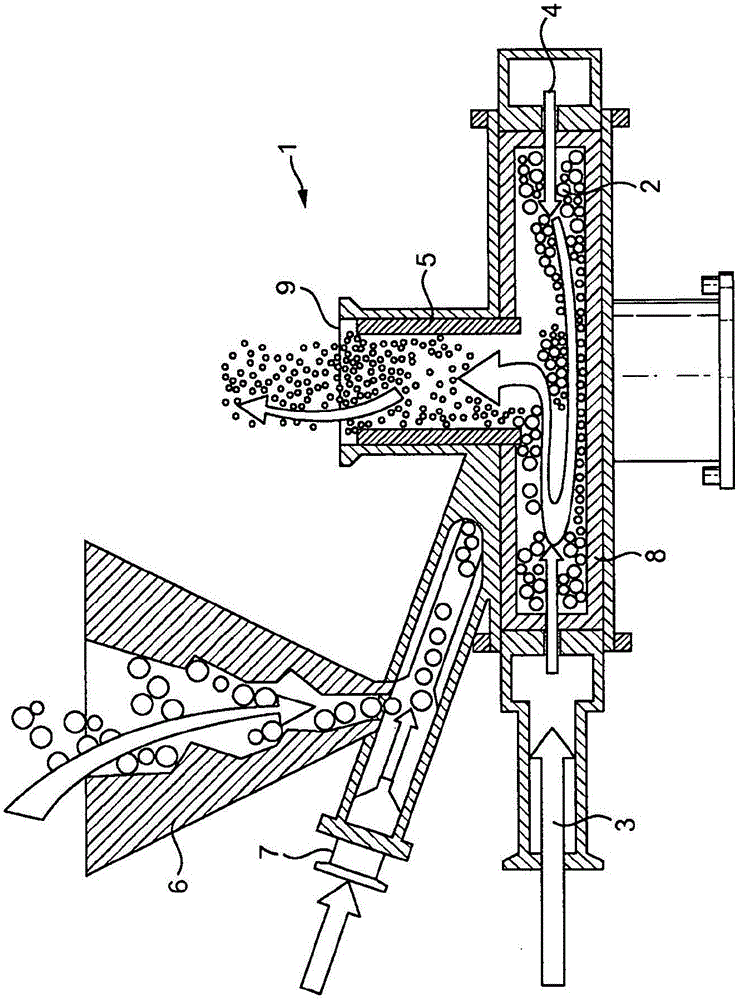
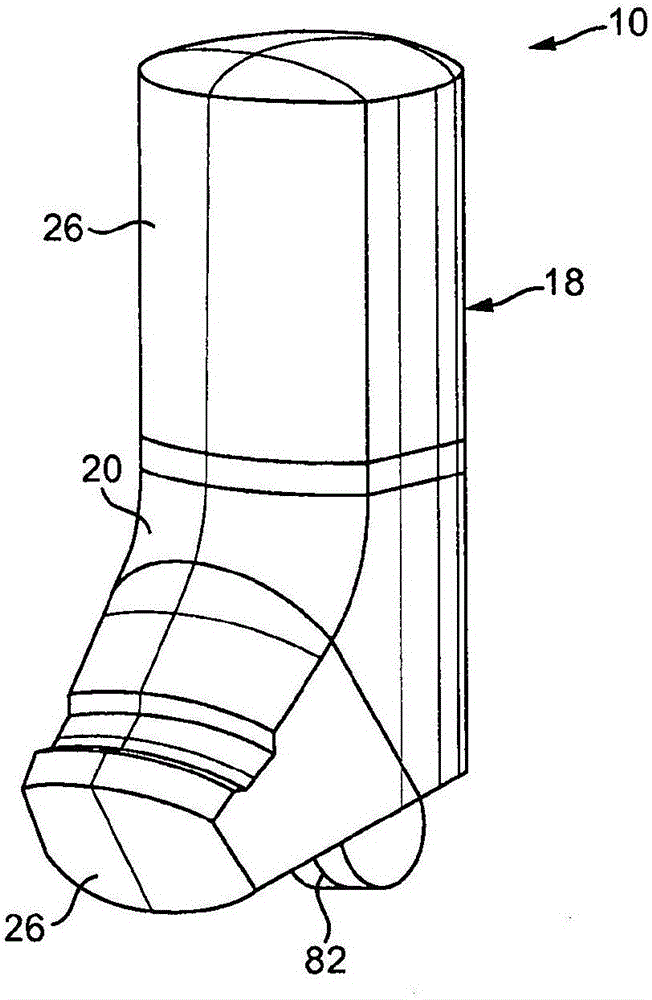
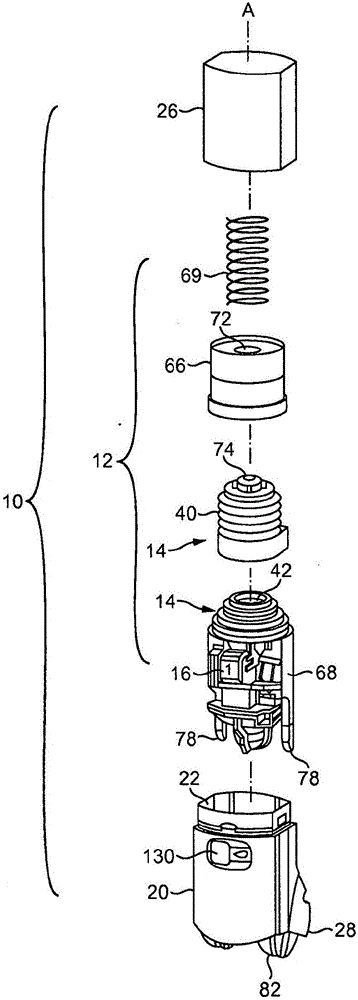
Inhalable medicaments
A technology for drugs and inhalers, applied in the field of inhalable drugs, can solve problems such as narrow treatment window and cardiac side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0120] Two samples of formoterol fumarate dihydrate were micronized by jet milling. These two lots are designated by codes 7544MA (normal mill) and 7544MO (invention). Micronization conditions are given in Table 1.
[0121] Table 1. Micronization conditions
[0122]
[0123]The process for Lot 7544MO used a lower milling pressure and higher feed rate than Lot 7544MA. Therefore, the process for batch 7544MO utilized lower energy to micronize formoterol than batch 7544MA. This is why the batches made from the 7544MO treatment had a consistently higher d90 diameter than the batches made from the 7544MA treatment.
[0124] The particle size of the two batches was measured using laser light scattering and dry particle dispersion methods, e.g. in air, e.g. by Sympatec HELOS / BF equipped with a RODOS disperser, the results are given in Table 2 and Figure 24 given in.
[0125] Table 2. Particle Sizes of Formoterol Batches
[0126]
[0127] The particle size distribution of...
example 2
[0129] Pharmacokinetic (PK) clinical studies were performed. PK studies utilize a stepwise approach to assess multiple key formulation parameters, metered dose (dose cup volume of device), formulation mixing intensity, drug particle size, and lactose particle size. PK studies were performed on the medium strength product (160 / 4.5 μg). Batch A contained Formoterol 7544MA and Batch B contained Formoterol 7544MO. The inhaler, budesonide and lactose were the same for batch A and batch B.
[0130] exist Figure 25 The key findings of the PK study are highlighted in . The data show that the coarser particle size formoterol significantly lowers Cmax, ie by more than 20%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com