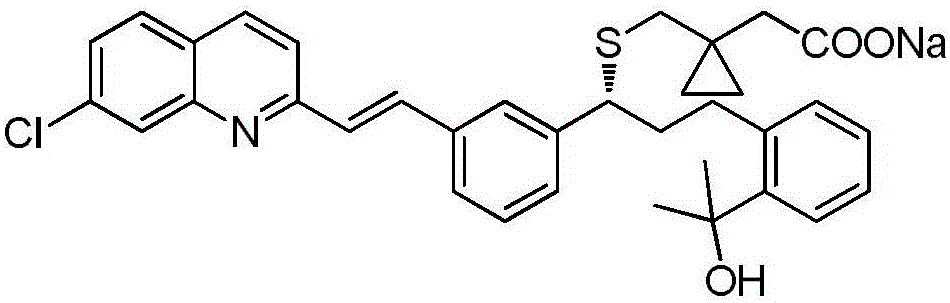
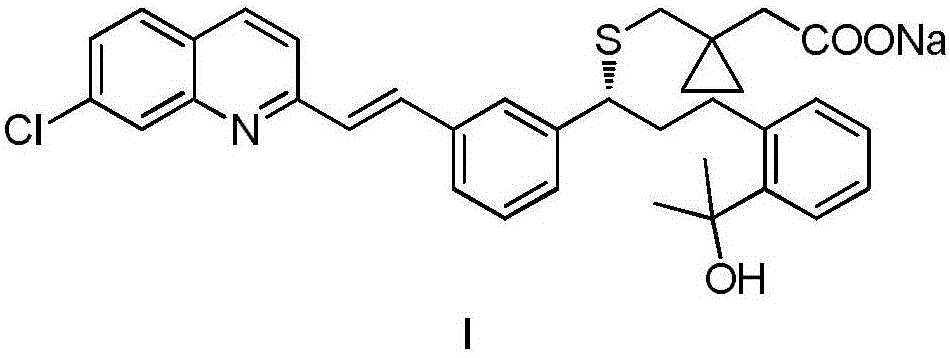
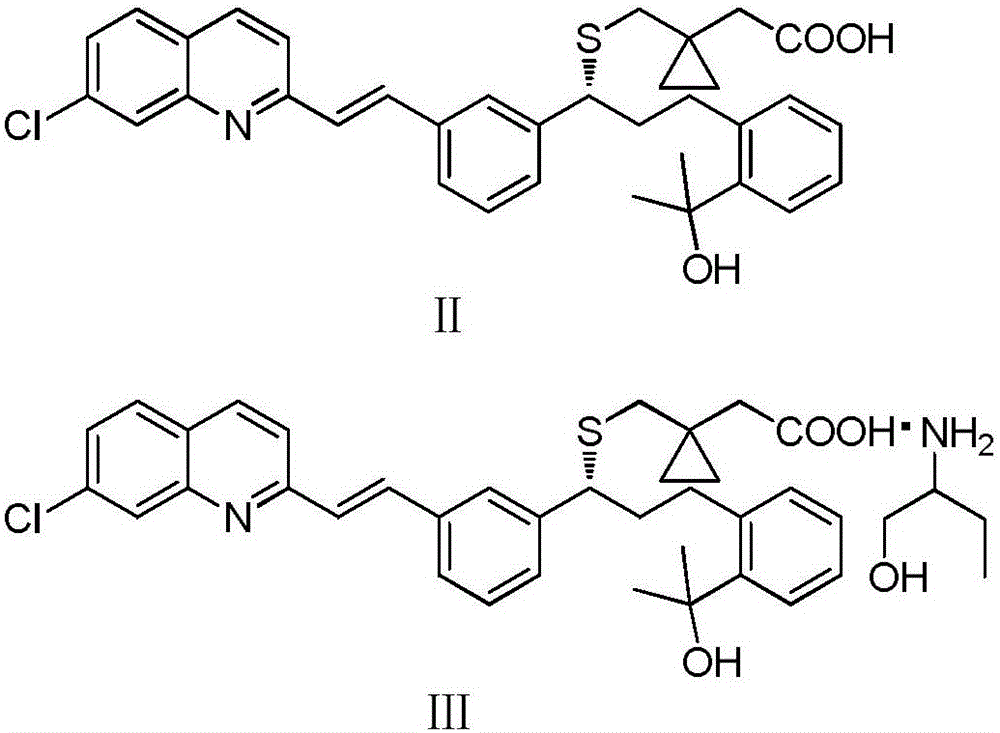
Preparation method of montelukast sodium
A technology of montelukast sodium and montelukast acid, which is applied in the field of chemical pharmaceuticals, can solve the problems of complex operation and low efficiency of impurity removal, and achieve the effect of reducing the content of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: a kind of preparation method of montelukast sodium, specifically as follows:
[0035] A. Add 50g of crude montelukast acid, 250mL of ethyl acetate, and 9.96mL of 2-amino-1-butanol to a 500mL three-necked flask in sequence, stir to dissolve, control the temperature at 15-25°C and stir for 5 hours, suction filter, 50 After vacuum drying at ℃ for 6 hours, montelukast acid 2-amino-1-butoxide was obtained with a yield of 92%.
[0036] B. Add 50g of montelukast acid 2-amino-1-butoxide and 400mL of toluene to a 1000mL three-necked flask in turn, reflux until dissolved, lower the temperature, control the temperature at 5-10°C, crystallize for 5 hours, and filter with suction. Vacuum drying at 60° C. for 8 hours gave montelukast acid 2-amino-1-butoxide with a yield of 95%.
[0037] C. Add 50g of montelukast acid 2-amino-1-butoxide and 400mL of dichloromethane to a 1000mL three-necked flask successively, and add aqueous acetic acid solution (10mL of acetic acid, di...
Embodiment 2
[0039] Embodiment 2: a kind of preparation method of montelukast sodium, specifically as follows:
[0040] A. Add 50g of crude montelukast acid, 350mL of ethyl acetate, and 8.3mL of 2-amino-1-butanol to a 500mL three-neck flask in sequence, stir to dissolve, control the temperature at 15-25°C for 4 hours, and suction filter, 50 °C and vacuum dried for 6 hours to obtain montelukast acid 2-amino-1-butoxide with a yield of 90%.
[0041] B. Add 50g of montelukast acid 2-amino-1-butoxide and 700mL of toluene to a 1000mL three-necked flask in turn, reflux until dissolved, lower the temperature, control the temperature at 20-25°C, crystallize for 5 hours, and filter with suction. After vacuum drying at 60°C for 8 hours, montelukast acid 2-amino-1-butoxide was obtained with a yield of 92.5%.
[0042] C. Add 50g of montelukast acid 2-amino-1-butoxide and 500mL of dichloromethane to a 1000mL three-necked flask successively, and add aqueous acetic acid solution (10mL of acetic acid, dil...
Embodiment 3
[0044] Embodiment 3: a kind of preparation method of montelukast sodium, specifically as follows:
[0045] A. Add 50g of crude montelukast acid, 500mL of ethyl acetate, and 20.74mL of 2-amino-1-butanol to a 500mL three-necked flask in sequence, stir to dissolve, control the temperature at 15-25°C and stir for 5 hours, then suction filter, 50 After vacuum drying at °C for 6 hours, montelukast acid 2-amino-1-butoxide was obtained with a yield of 86.6%.
[0046] B. Add 50g of montelukast acid 2-amino-1-butoxide and 400mL of toluene to a 1000mL three-necked flask in sequence, reflux until dissolved, lower the temperature, control the temperature at 20-30°C, crystallize for 20 hours, and filter with suction. After vacuum drying at 60°C for 8 hours, montelukast acid 2-amino-1-butoxide was obtained with a yield of 92.1%.
[0047] C. Add 50g of montelukast acid 2-amino-1-butoxide and 600mL of dichloromethane to a 1000mL three-necked flask successively, and add aqueous acetic acid sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com