A method for improving the reporting rate of clinical adverse drug reactions
A technology for adverse reactions and drugs, applied in the field of medical systems, can solve problems such as low reporting rates, and achieve the effects of reducing non-reporting, reducing the time limit of lesions, and improving the pass rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] A method for improving the reporting rate of clinical adverse drug reactions, such as figure 1 As shown, the method includes the following steps,
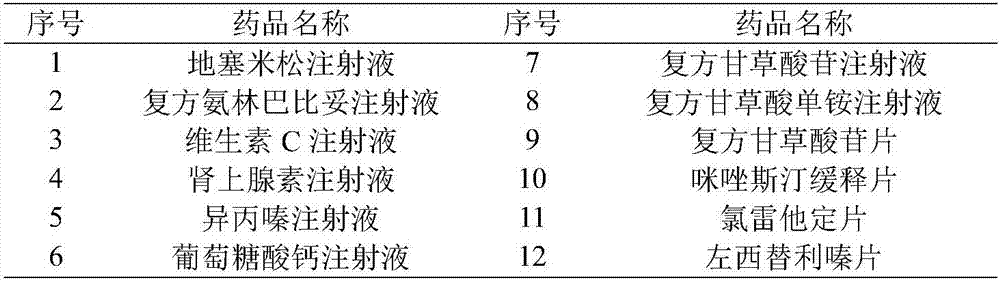
[0024] Step 1. Determine the first-aid drugs and anti-allergic drugs used for ADR adverse drug reactions
[0025] First, the ADR exclusive central processor is connected to the hospital's internal LAN through the CAN bus, and the name of the drug is retrieved from the emergency drug and anti-allergic drug database.
[0026] Secondly, the clinical use data of emergency medicine and antiallergic medicine were retrieved from the case database,
[0027] Input the obtained specific data into the ADR medication situation analysis module again,
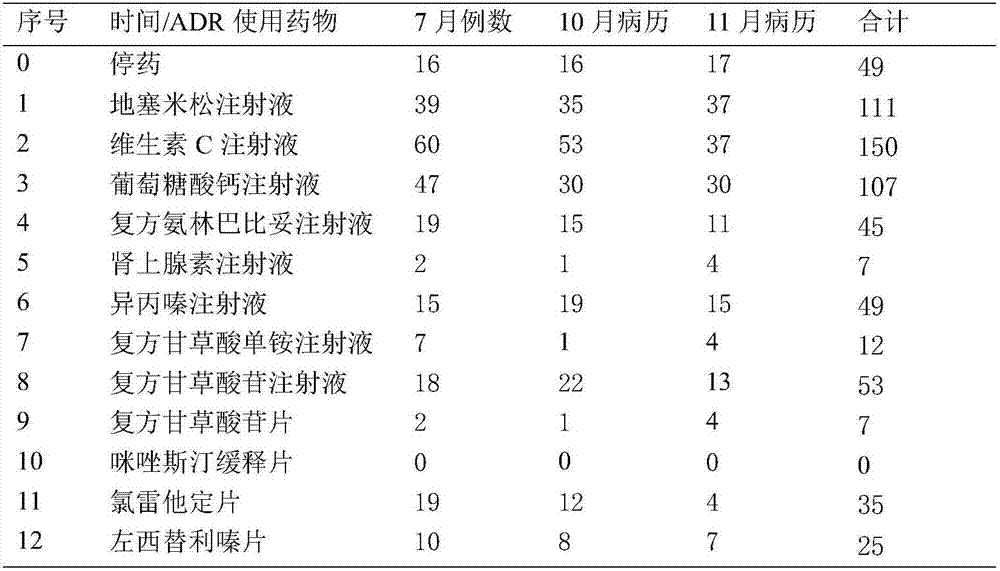
[0028] Finally, dexamethasone injection, vitamin C injection, calcium gluconate injection and loratadine tablets were obtained as monitoring drugs;
[0029] Step 2. Front-line doctors input the clinical use data of dexamethasone injection, vitamin C injection, calcium gluconate injectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com