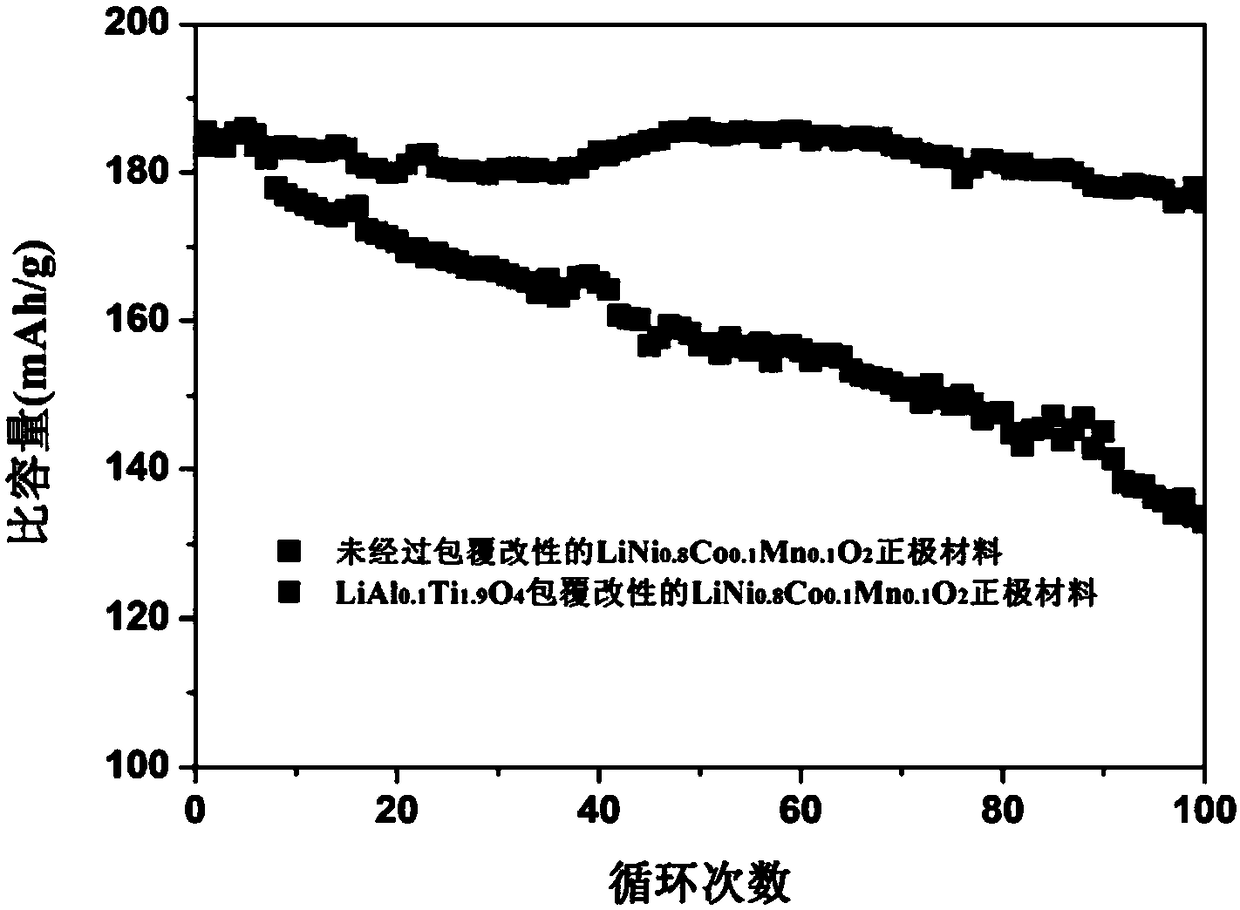
Preparation method and application of metal-doped spinel structure fast ion conductor coated nickel-containing positive electrode material
A technology of spinel structure and metal doping, applied in the preparation of lithium-ion batteries, preparation of metal-doped spinel structure fast ion conductors coated with nickel-containing positive electrode materials, field of modified lithium-ion battery positive electrode materials , can solve the problems of high cost, complex operation of fast ion conductors, and difficulty in industrial production, and achieve the effect of preventing dissolution loss, facilitating large-scale industrial production, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) According to LiAl 0.1 Ti 1.9 O 4 The coating mass percentage of the fast ion conductor relative to the positive electrode material is 0.5%. A quantitative amount of titanium isopropoxide is fully dispersed in anhydrous ethanol for 0.5 h using a mechanical stirring method or an ultrasonic method.
[0038] (2) The titanium isopropoxide dispersion in step (1) is heated in a water bath at 80°C, and the layered LiNi 0.8 Co 0.1 Mn 0.1 O 2 The positive electrode material was added to the titanium isopropoxide dispersion, and stirred and dispersed for 0.5 h.
[0039] (3) Dissolve the aluminum-containing metal salt in deionized water according to 1.4% of the atomic percentage of the doped metal element in the fast ion conductor, and add it to the titanium isopropoxide dispersion in step (2), and Continue stirring for 1.0h.
[0040] (4) Add the mixture obtained in step (3) to the reaction kettle, keep it at 80°C for 24h in a blast drying box, cool to room temperature naturally, wash...
Embodiment 2
[0048] (1) According to LiCe 0.05 Ti 1.95 O 4 The coating mass percentage of the fast ion conductor relative to the positive electrode material is 1.5%. A quantitative amount of titanium isopropoxide is fully dispersed in anhydrous ethanol for 0.2h by mechanical stirring method or ultrasonic method.
[0049] (2) The titanium isopropoxide dispersion in step (1) is heated in a water bath at 50°C, and the layered LiNi 0.85 Co 0.05 Mn 0.1 O 2 The positive electrode material was added to the titanium isopropoxide dispersion, and stirred and dispersed for 0.8 h.
[0050] (3) The cerium-containing metal salt is dissolved in deionized water according to 0.7% of the atomic percentage of the fast ion conductor occupied by the doped metal element, and added to the titanium isopropoxide dispersion in step (2), and Continue to stir for 1.5h.
[0051] (4) Add the mixture obtained in step (3) to the reaction kettle, keep it at 120°C for 48h in a blast drying box, cool it naturally to room temperatu...
Embodiment 3
[0056] (1) According to LiV 0.15 Ti 1.85 O 4 The coating mass percentage of the fast ion conductor relative to the positive electrode material is 2.0%. A quantitative amount of titanium isopropoxide is fully dispersed in absolute ethanol for 1.5 hours by mechanical stirring method or ultrasonic method.
[0057] (2) The titanium isopropoxide dispersion in step (1) is heated in a water bath at 50°C, and the layered LiNi 0.5 Co 0.2 Mn 0.3 O 2 The positive electrode material was added to the titanium isopropoxide dispersion, and stirred and dispersed for 4.0 h.
[0058] (3) The vanadium-containing metal salt is dissolved in deionized water according to 2.0% of the atomic percentage of the fast ion conductor occupied by the doped metal element, and added to the titanium isopropoxide dispersion in step (2), and Continue stirring for 1.0h.
[0059] (4) Add the mixture obtained in step (3) to the reaction kettle, keep it at 140°C for 36 hours in a blast drying box, cool it naturally to room ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com