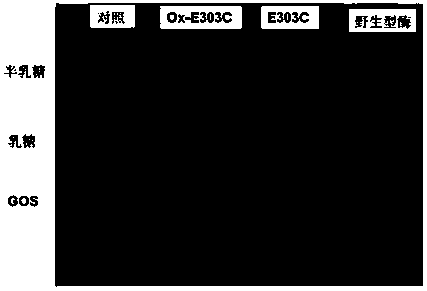
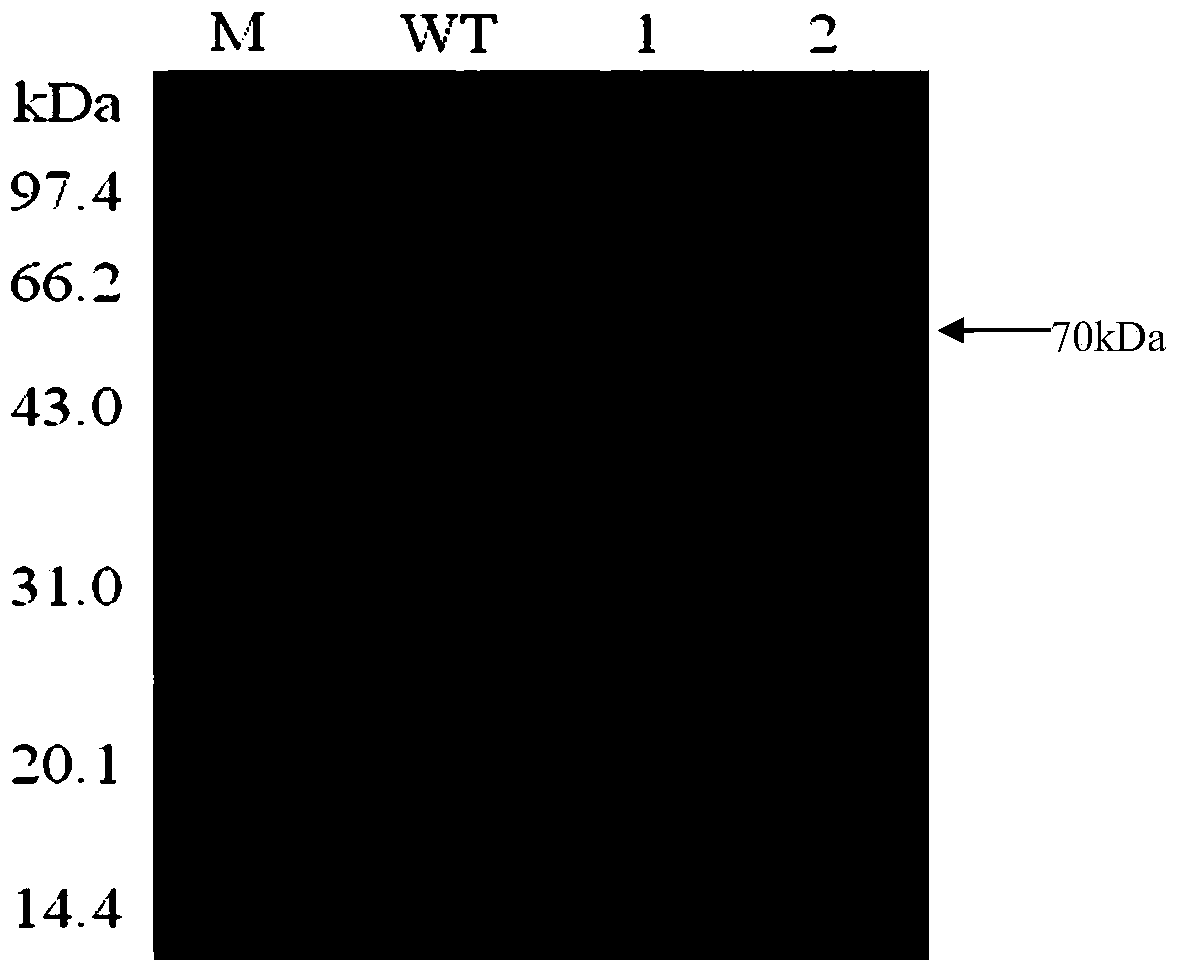
A thermostable β-galactosidase mutant with transglycosidic activity and its preparation method
A technology for galactosidase and transglycosidase activity, which is applied in the field of preparation of heat-resistant beta-galactosidase mutants with transglycosidase activity, can solve problems such as unverified catalytic changes, and achieves speeding up functional evolution efficiency and improving The amount of synthesis, the effect of avoiding hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1. Construction of E303C mutant
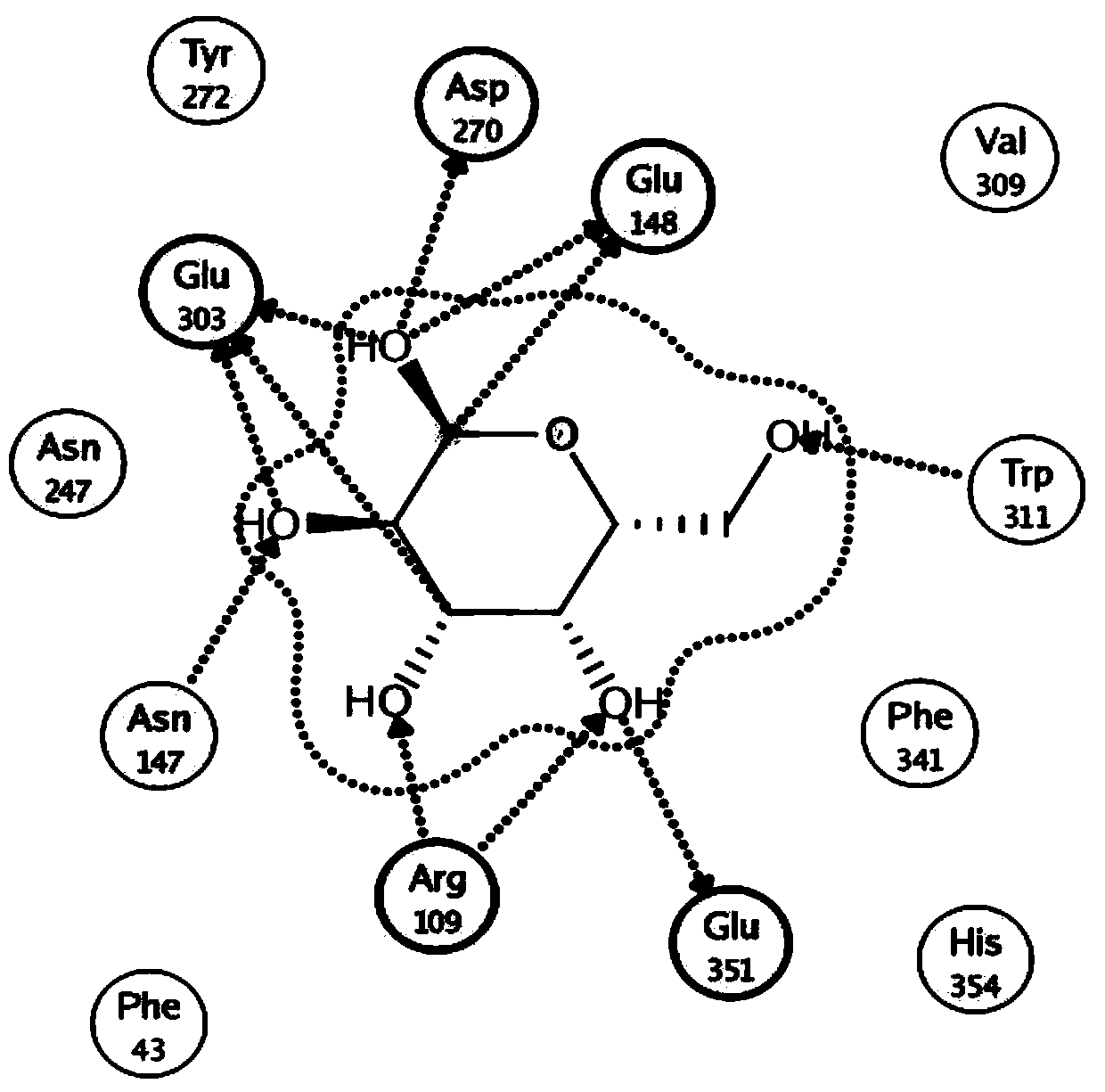
[0043] Through the molecular simulation of BgaB and the docking of galactose and lactose molecules with the enzyme, it is predicted that the key sites of β-galactosidase catalysis and the key amino acid sites involved in substrate binding are Glu148 and Glu303 (such as figure 1 ). Among them, Glu303 is the homologous site of nucleophile amino acid according to sequence alignment. In hydrolases with maintenance and inversion catalytic mechanisms, the catalytic residue plays a key role in the activity of the enzyme. Therefore, it is speculated that the Glu303 site may have a regulatory effect on the catalytic activity of β-galactase BgaB, so the Glu303 amino acid site was taken as the target site for research and transformation.
[0044] In this regard, the plasmid pKK223-3-bgaB containing the heat-resistant β-galactosidase gene bgab derived from Bacillus stearothermophilus was used as a template (the plasmid was provided by the Cultur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com