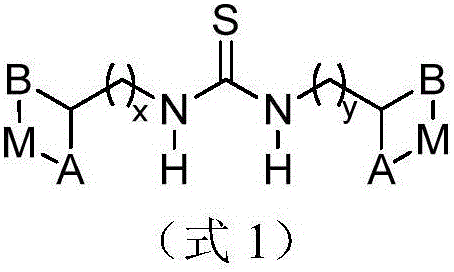
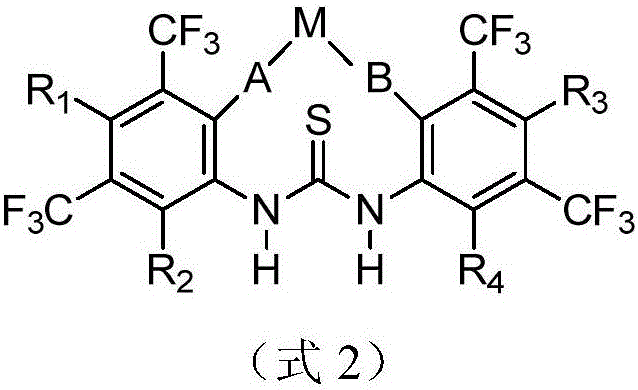
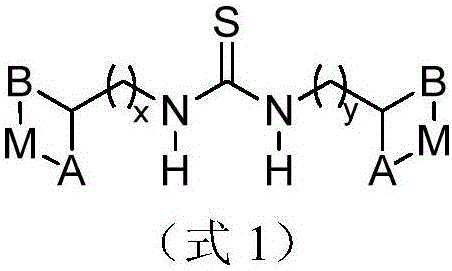
Metal complex for catalysis of caprolactone polymerization
A metal complex, caprolactone technology, applied in the direction of organic chemistry, etc., can solve the problem that metal catalysts have not been reported, and achieve the effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] In Examples 11-15, the metal complexes in the above-mentioned Example 3 were used as catalysts, and bulk polymerization was carried out under different temperature conditions. 5.7 g (50 mmol) of ε-caprolactone were respectively placed in a 50 mL flask, and 0.2 mmol of the metal complex in Example 1 and 0.1 mmol of benzyl alcohol were added. Heating in an oil bath at 5 temperatures, constant temperature reaction for 1 hour, cooling, sampling and analysis, the conversion rate was measured by liquid chromatography, and the molecular weight was measured by GPC (polystyrene as a standard sample). The experimental results of Examples 11-15 are shown in Table 2.
[0047] The ε-caprolactone monomer described in Examples 11-15 is a product with higher purity after rectification in the production process of caprolactone. It can be seen that the high reaction temperature is conducive to the acceleration of the reaction, but the molecular weight distribution gradually increases, a...
Embodiment 3
[0062] Examples 31-34 use the metal complex of the above-mentioned Example 3 as a catalyst to catalyze the bulk polymerization of 4 different cyclic esters. 4 kinds, 5g of cyclic esters were respectively placed in 50mL flasks, 1mg of the polymerization inhibitor in Example 3 was added, heated in an oil bath at 120°C, reacted for 4 hours, cooled, and the content of cyclic esters was analyzed by sampling. Calculate the cyclic ester conversion rate (ie polymerization rate) with this, the experimental result of embodiment 31-34 is shown in Table 5, and thiourea metal salt is lower to other cyclic esters except valerolactone, lactide catalytic activity, may be different from Ring monomer ring tension dependent.
[0063] Polymerization of different cyclic ester monomers in table 5
[0064]
[0065]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com