Intumescent flame retardant containing polyhydroxy groups and phosphorous triazine ring and preparation method
An intumescent flame retardant, technology containing polyhydric groups, is applied in chemical instruments and methods, organic chemistry, compounds of Group 5/15 elements of the periodic table, etc., and can solve problems such as high environmental and human hazards, large quantities, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
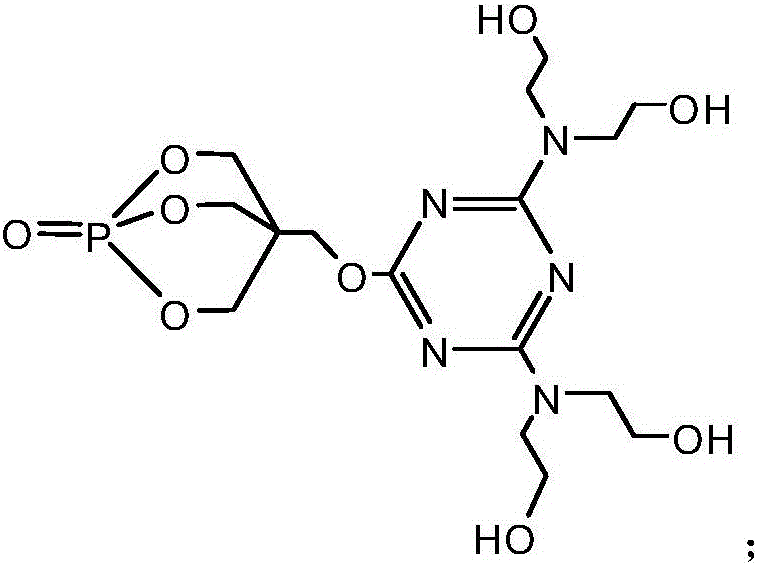
[0036] Add 18.45g of cyanuric chloride and 100ml of acetone solution (the volume ratio of acetone and water is 1:1) in the 1L reaction flask equipped with reflux condenser, constant pressure dropping funnel, stirrer and thermometer, fully stir, Make the cyanuric chloride disperse evenly, then under the condition of 0℃, add 18g of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2. 2] Octane and NaHCO 3 solution (8.4g NaHCO 3 dissolved in 100ml distilled water), after the dropwise addition was completed, the reaction was carried out at 0° C. for 3 hours. Then raise the temperature to 40°C, add 100ml of acetone solution, and then evenly and slowly add 10.5g of diethanolamine and NaHCO 3 solution (8.4g NaHCO 3 Dissolve in 100ml distilled water), keep the temperature at 40°C, after the dropwise addition is complete, react under this condition for 8 hours. Then heat up to 60°C, distill off the acetone, then continue to heat up to 85°C, and evenly and slowly add 13g of diethan...
Embodiment 2
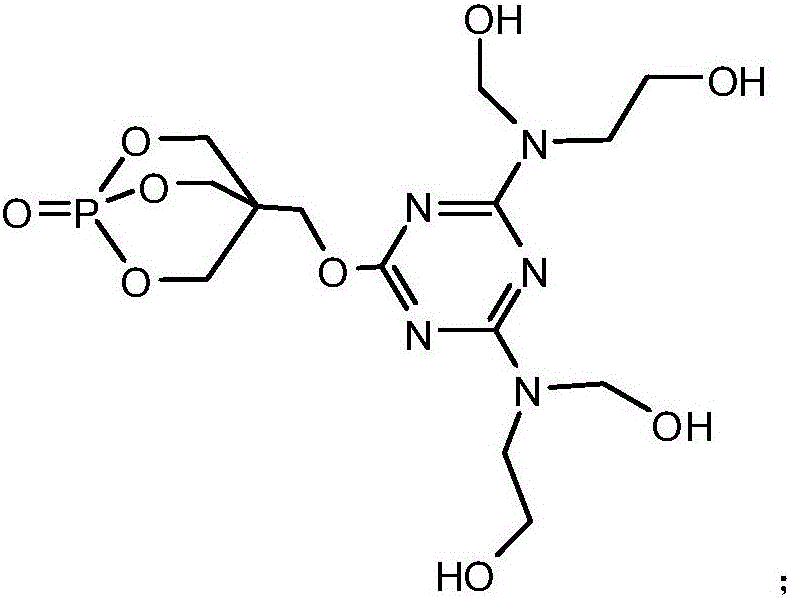
[0040] Add 18.45g of cyanuric chloride and 100ml of acetone solution (the volume ratio of acetone and water is 1:1) in the 1L reaction flask equipped with reflux condenser, constant pressure dropping funnel, stirrer and thermometer, fully stir, Make the cyanuric chloride disperse evenly, then under the condition of 0℃, add 18g of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2. 2] octane and triethylamine solution (10.1g triethylamine dissolved in 100ml distilled water), after the dropwise addition, react at 0°C for 3 hours. Then raise the temperature to 40°C, add 100ml of acetone solution, then evenly and slowly add 9.1g of 2-hydroxymethylaminoethanol and triethylamine solution (10.1g of triethylamine is dissolved in 100ml of distilled water) dropwise in the reaction flask, Keep the temperature at 40°C, and react under this condition for 8 hours after the dropwise addition is complete. Then heat up to 60°C, distill out acetone, then continue to heat up to 85°C, evenly a...
Embodiment 3
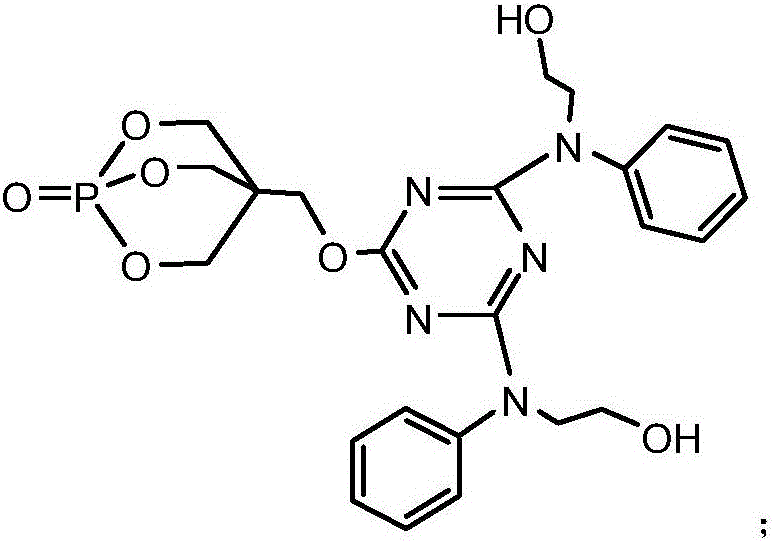
[0044] Add 18.45g of cyanuric chloride and 100ml of acetone solution (the volume ratio of acetone and water is 1:1.5) in the 1L reaction flask equipped with reflux condenser, constant pressure dropping funnel, stirrer and thermometer, fully stir, Disperse cyanuric chloride evenly, and then add 18g of 1-oxyphospha-4-hydroxymethyl-2,6,7-trioxabicyclo[2.2 .2] Octane and NaHCO 3 solution (8.4g NaHCO 3 dissolved in 100ml of distilled water), after the dropwise addition was completed, the reaction was carried out at -5°C for 4 hours. Then raise the temperature to 50°C, add 100ml of acetone solution, then evenly and slowly add 13.7g of 2-phenyldiethanolamine and NaHCO3 solution (8.4g of NaHCO3 dissolved in 100ml of distilled water) dropwise to the reaction flask, keeping the temperature at 50°C , After the dropwise addition was completed, the reaction was carried out under this condition for 6 hours. Then heat up to 60°C, distill off the acetone, then continue to heat up to 100°C,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| limiting oxygen index | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
| limiting oxygen index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com