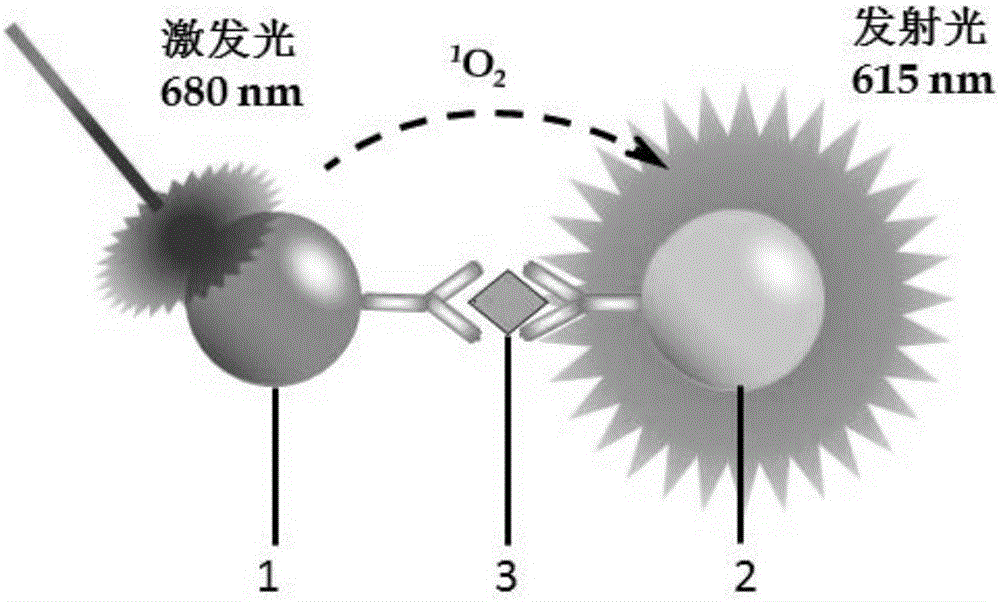
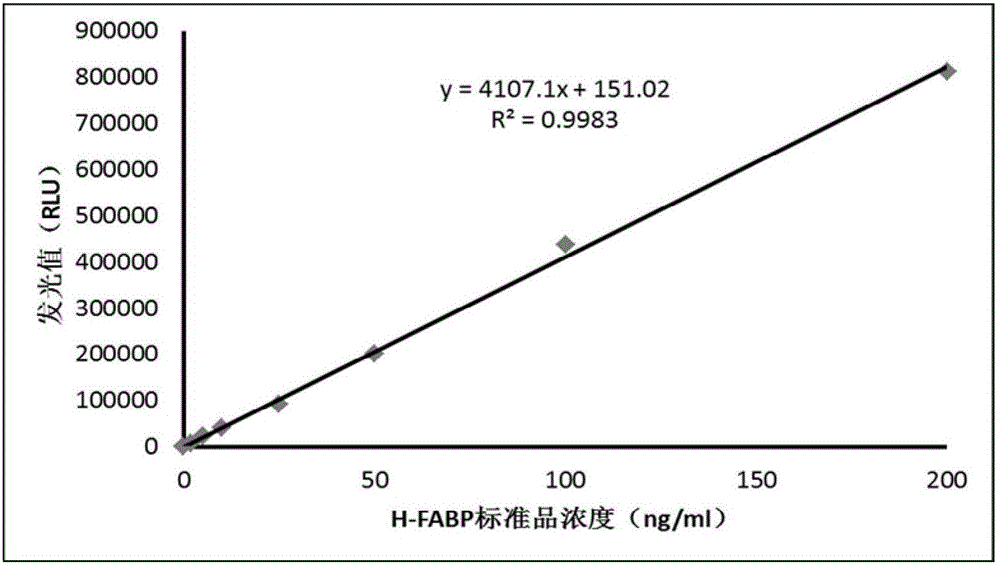
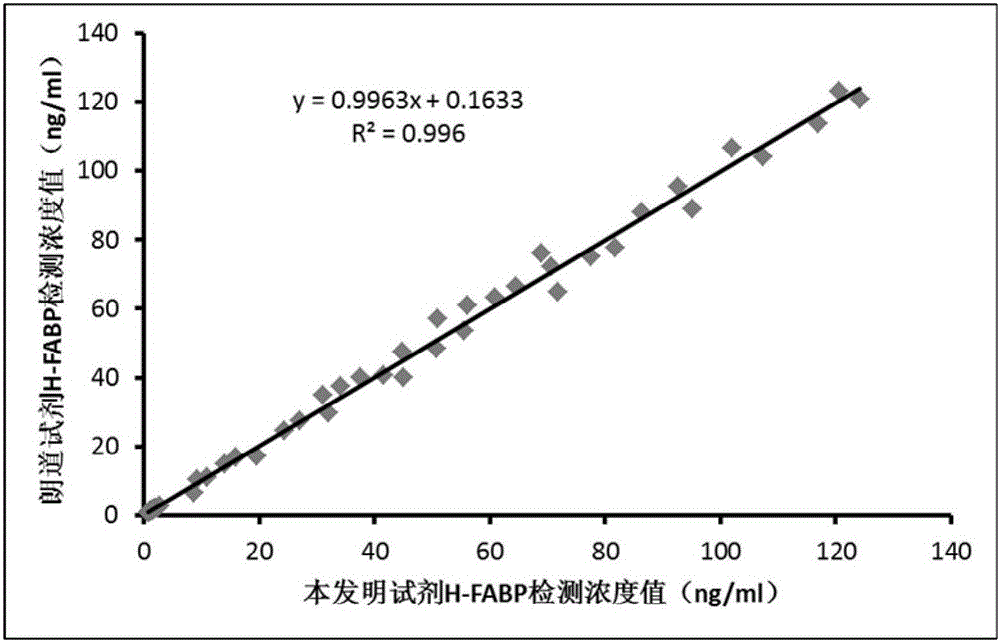
One-step homogeneous-phase H-FABP detection kit and preparation and use method thereof
A kit and chemiluminescent detection technology, applied in the field of one-step homogeneous H-FABP detection kits, can solve the problems of inability to balance sensitivity and linearity, reduce detection repeatability, etc., and achieve clinical detection convenience, large market value, The effect of high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Preparation of anti-H-FABP antibody-coupled luminescent microspheres:
[0045] (1) Add 0.1 mg of anti-H-FABP monoclonal antibody to an ultrafiltration tube, centrifuge for 10 minutes, wash with HEPES buffer (pH7.4) for 6 times, and dilute the antibody to 1 mg / ml for use. Wash 0.1 mg of luminescent microspheres twice with HEPES buffer (pH7.4), centrifuge, and remove the supernatant;
[0046] (2) Add the antibody solution to the luminescent microspheres and resuspend;
[0047] (3) Add 2.0μl 10% Tween 20, 8μl 400mM NaCNBH 3 , add HEPES buffer to make up the volume to 200 μl, shake and react at 37°C for 24 hours;
[0048] (4) Prepare 65mg / ml CMO with 800mM NaOH, add 8μl of the solution to the reaction system; shake and react at 37°C for 0.5 hours, centrifuge, and remove the supernatant;
[0049] (5) Add 150 μl Tris-HCl (pH8.0) buffer to resuspend, centrifuge, remove supernatant, repeat once;
[0050] (6) After the last centrifugation, resuspend the microspheres with a m...
Embodiment 2
[0062] Preparation of anti-H-FABP antibody-coupled luminescent microspheres:
[0063] (1) Add 0.1 mg of anti-H-FABP polyclonal antibody to an ultrafiltration tube, centrifuge for 10 minutes, wash with HEPES buffer (pH7.4) for 6 times, and dilute the antibody to 1 mg / ml for use. Wash 10 mg of luminescent microspheres twice with HEPES buffer (pH7.4), centrifuge, and remove the supernatant;
[0064] (2) Add the antibody solution to the luminescent microspheres and resuspend;
[0065] (3) Add 3μl 10% Tween 20, 12μl 400mM NaCNBH 3 , add HEPES buffer to make up the volume to 200 μl, shake and react at 37°C for 48 hours;
[0066] (4) Prepare 65 mg / ml CMO with 800 mM NaOH, add 12 μl of the solution to the reaction system; shake and react at 37 ° C for 1.5 hours, centrifuge, and remove the supernatant;
[0067] (5) Add 250 μl Tris-HCl (pH8.0) buffer to resuspend, centrifuge, remove supernatant, repeat once;
[0068] (6) After the last centrifugation, resuspend the microspheres with...
Embodiment 3
[0080] (1) Add 0.1 mg of anti-H-FABP monoclonal antibody to an ultrafiltration tube, centrifuge for 10 minutes, wash with HEPES buffer (pH7.4) for 6 times, and dilute the antibody to 1 mg / ml for use. Wash 1 mg of luminescent microspheres twice with HEPES buffer (pH7.4), centrifuge, and remove the supernatant;
[0081] (2) Add the antibody solution to the luminescent microspheres and resuspend;
[0082] (3) Add 2.5μl 10% Tween 20, 10μl 400mM NaCNBH 3 , add HEPES buffer to make up the volume to 200μl, shake and react at 37°C for 36 hours;
[0083] (4) Prepare 65 mg / ml CMO with 800 mM NaOH, add 10 μl of the solution to the reaction system; shake and react at 37 ° C for 1 hour, centrifuge, and remove the supernatant;
[0084] (5) Add 200 μl Tris-HCl (pH8.0) buffer to resuspend, centrifuge, remove supernatant, repeat once;
[0085] (6) After the last centrifugation, resuspend the microspheres with a mixture of PBS buffer (pH7-8) and bovine serum albumin, with a final concentrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Analytical sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com