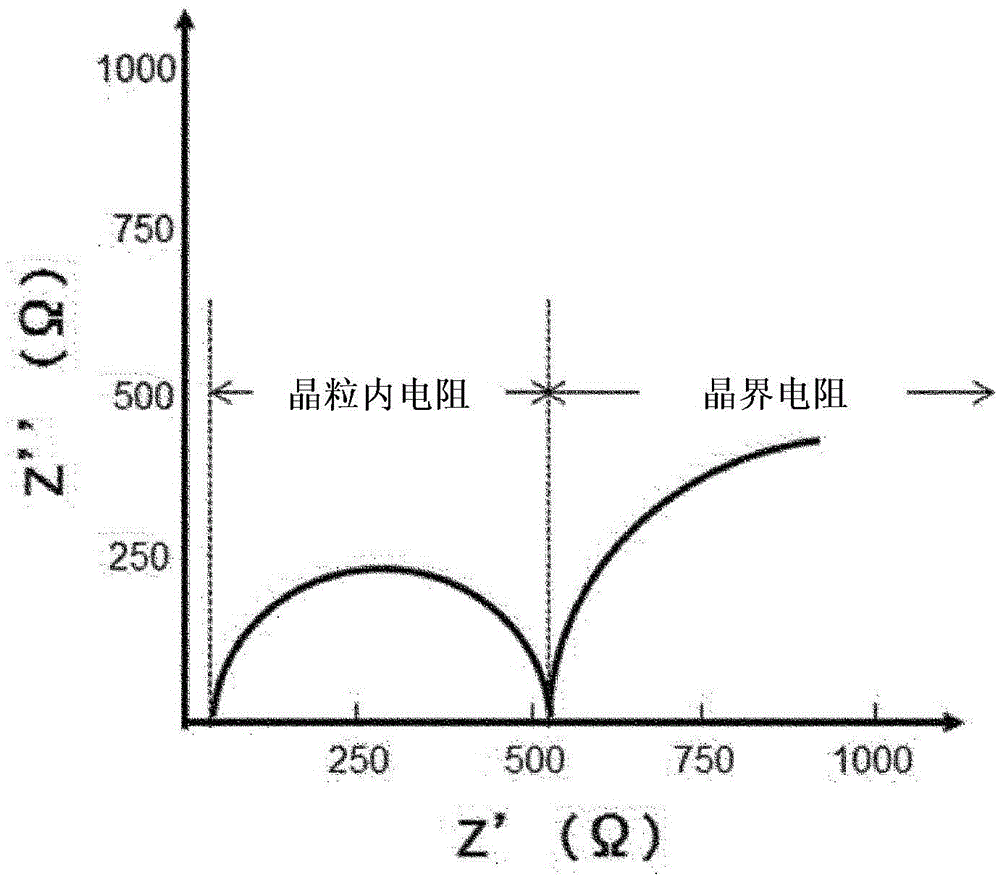
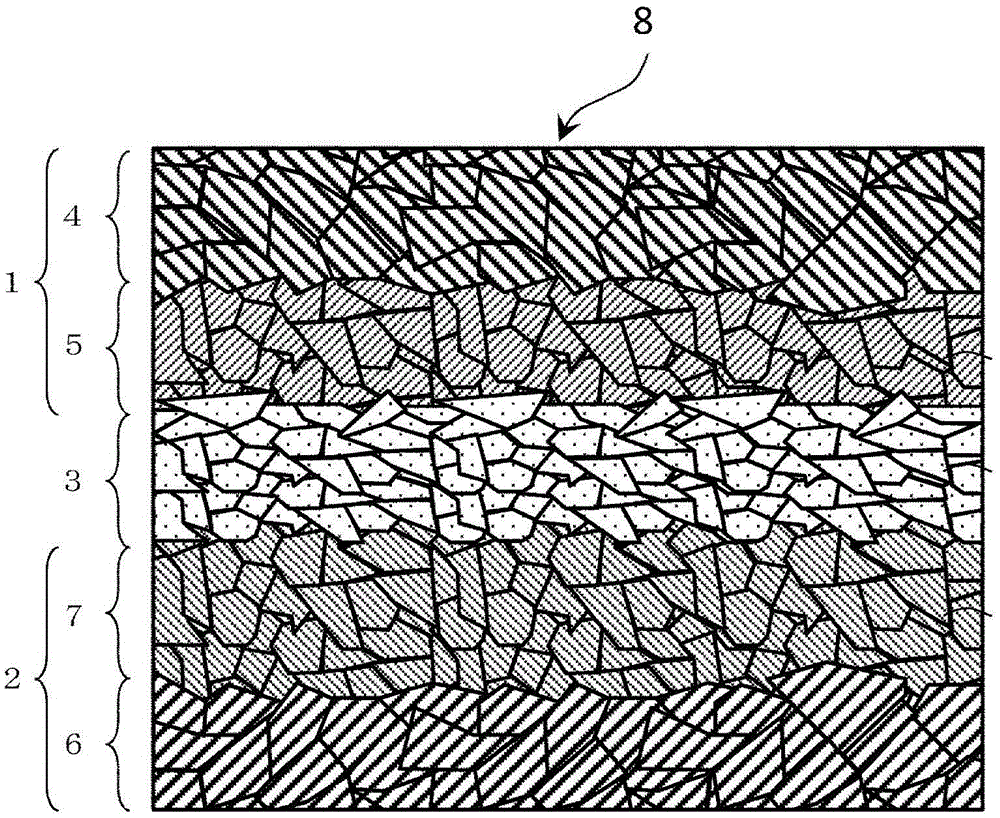
Li-ion conductive oxide ceramic material with garnet type crystal structure or crystal structure similar to garnet type
An oxide ceramic, garnet-type technology, applied in electrochemical generators, electrical components, circuits, etc., can solve the problem that all-solid-state lithium-ion secondary batteries are not practical.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~ Embodiment 10
[0087] In order to verify the effect of the present embodiment, as an example of a lithium ion conductive oxide ceramic material having a garnet type or a garnet-like crystal structure, proposed are respectively substituted Li 7.10 La 3.00 (Zr 1.90 A 0.10 )O 12 (A=Y, Nd, Gd, Ho, Yb) composition (Example 1-Example 5), further adding 1.0wt% Al to each composition 2 o 3 The composition of (embodiment 6-embodiment 10). Li was used in the starting material 2 CO 3 、La(OH) 3 , ZrO 2 , Y 2 o 3 、Nd 2 o 3 、Gd 2 o 3 、Ho 2 o 3 , Yb 2 o 3 and Al 2 o 3 . First, starting materials were weighed so as to have a stoichiometric ratio, and mixed and pulverized in ethanol with a ball mill (120 rpm / zirconia ball) for 16 hours. After the mixed powder of the starting material was separated from the balls and ethanol, it was calcined in an alumina crucible at 900° C. in the air atmosphere for 5 hours. Then, for mixing, the calcined powder was treated with a ball mill (120 rpm / zi...
Embodiment 11~ Embodiment 26
[0089] In addition, it is proposed to substitute Li for Zr with A, respectively 7.35 La 3.00 (Zr 1.65 A 0.35 )O 12 , (A=Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu) composition (Example 11-18) and further added 1.0wt% Al to each composition 2 o 3 The composition of (embodiment 19-embodiment 26). The starting material used Li 2 CO 3 、La(OH) 3 , ZrO 2 、Gd 2 o 3 , Tb 2 o 3 、Dy 2 o 3 、Ho 2 o 3 、Er 2 o 3 、Tm 2 o 3 , Yb 2 o 3 、Lu 2 o 3 and Al 2 o 3. First, the starting materials were weighed until the stoichiometric ratio was reached, and mixed and pulverized in ethanol with a ball mill (120 rpm / zirconia ball) for 16 hours. After the mixed powder of the starting material was separated from the balls and ethanol, it was calcined in an alumina crucible at 900° C. in the air atmosphere for 5 hours. Then, for mixing, the calcined powder was treated with a ball mill (120 rpm / zirconia ball) in ethanol for 16 hours. After the pulverized powder was separated from the balls ...
Embodiment 27~ Embodiment 29
[0091] In addition, to Li 7.05 La 3.00 (Zr 1.95 Gd 0.05 )O 12 , Li 7.25 La 3.00 (Zr 1.75 Gd 0.25 )O 12 , Li 7.50 La 3.00 (Zr 1.50 Gd 0.50 )O 12 Add 1.0wt% Al respectively 2 o 3 . The initial raw material uses Li 2 CO 3 、La(OH) 3 , ZrO 2 、Gd 2 o 3 and Al 2 o 3 . First, the starting materials were weighed so that the stoichiometric ratio was obtained, and mixed and pulverized in ethanol with a ball mill (120 rpm / zirconia ball) for 16 hours. After the mixed powder of the starting material was separated from the balls and ethanol, it was calcined in an alumina crucible at 900° C. in an air atmosphere for 5 hours. Then, for mixing, the calcined powder was treated with a ball mill (120 rpm / zirconia ball) in ethanol for 16 hours. After the pulverized powder was separated from the balls and ethanol and dried, the powder before main sintering was obtained. Next, an organic binder is added to these pre-sintered powders to produce pellets. The pellets were mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com