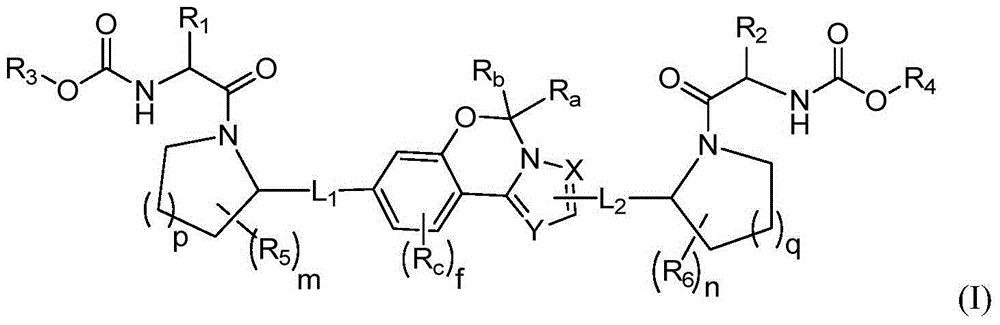
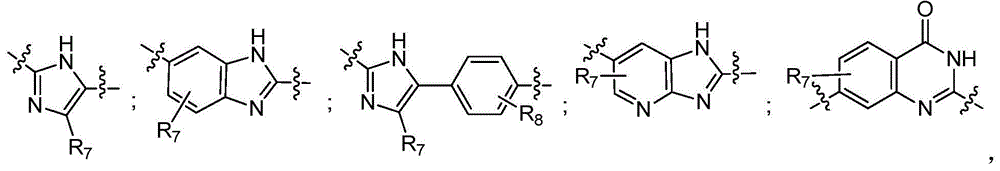
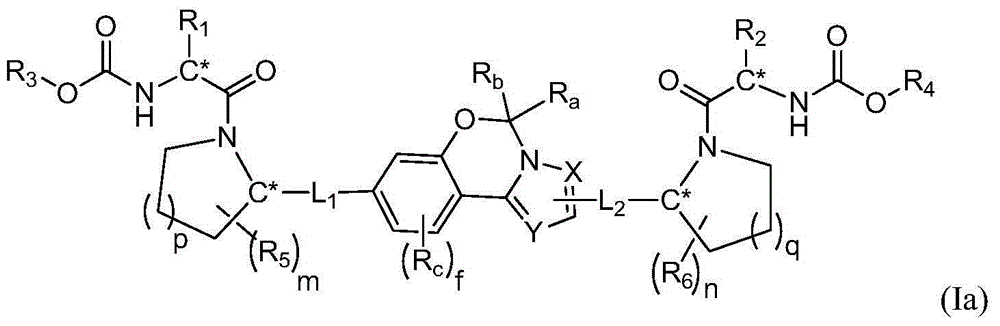
Fused tricyclic hepatitis virus inhibitor and application thereof
A compound, cycloalkyl technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Example 1 N-((2S)-1-((S)-2-(5-(3-(2-((2S)-1-((S)-2-methoxycarbonylamino-3-methyl ylbutyryl)pyrrol-2-yl)-1H-benzo[d]imidazol-6-yl)-5-phenyl-5H-benzo[e]imidazo[1,2-c][1,3 ]oxazin-8-yl)-1H-imidazol-2-yl)pyrrol-1-yl)-3-methyl-1-oxobutane-2-yl)methyl carbamate
[0140]
[0141] Step 1 Preparation of 5-bromo-2-(1H-imidazol-2-yl)phenol
[0142]
[0143] Weigh 11.0g of 4-bromo-2-hydroxybenzaldehyde in a dry 500mL single-necked bottle, add 200mL of methanol, add 40.0g of glyoxal (40%wt) at 0°C, continue to drop 50mL of ammonia (17%-22 %wt), stirring in an ice bath to naturally rise to room temperature, and reacting for 2 hours. After the reaction, the reaction solution was concentrated at 60°C and purified by column chromatography to obtain the title compound.
[0144] 1 H NMR: (500MHz, DMSO-d 6 )δ13.16-13.02 (brs, 2H), 7.80 (d, 1H), 7.28-7.25 (brs, 2H), 7.15-7.10 (m, 2H).
[0145] MS(ESI):[M+H] + =239.
[0146] Step 2 Preparation of 8-bromo-5-phenyl-5H-benzo[e]imid...
Embodiment 2
[0198] Example 2 N-((2S)-1-((S)-2-(5-(3-(2-((2S)-1-((S)-2-methoxycarbonylamino-3,3 -Dimethylbutyryl)pyrrol-2-yl)-1H-benzo[d]imidazol-6-yl)-5-phenyl-5H-benzo[e]imidazo[1,2-c][ 1,3]oxazin-8-yl)-1H-imidazol-2-yl)pyrrol-1-yl)-3,3-dimethyl-1-oxobutan-2-yl)methyl carbamate
[0199]
[0200] Compound (S)-2-(5-(3-(2-((2S)-pyrrol-2-yl)-1H-benzo[d]imidazol-6-yl)- 5-Phenyl-5H-benzo[e]imidazo[1,2-c][1,3]oxazin-8-yl)-1H-imidazol-2-yl)pyrrole and N-methoxycarbonyl- Using L-tert-leucine as the starting material, the title compound was prepared according to the method in Step 14 of Example 1.
[0201] 1 H NMR: (300MHz, DMSO-d 6 )δ12.23-12.28(brs,1H),11.80-11.83(brs,1H),8.16-8.19(m,1H),7.70-7.73(m,1H),6.98-7.63(m,14H),5.01- 5.20 (m, 2H), 4.20-4.23 (m, 2H), 3.73-3.82 (m, 4H), 3.68 (s, 6H), 1.98-2.13 (m, 8H), 0.83-0.96 (m, 18H).
[0202] MS(ESI):[M+H] + =911.
Embodiment 3
[0203] Example 3 N-((2S)-1-((S)-2-(5-(3-(2-((2S)-1-((S)-2-methoxycarbonylamino-3-methyl ylbutyryl)pyrrol-2-yl)-1H-benzo[d]imidazol-6-yl)-5-(4-tert-butylphenyl)-5H-benzo[e]imidazo[1,2 -c][1,3]oxazin-8-yl)-1H-imidazol-2-yl)pyrrol-1-yl)-3-methyl-1-oxobutan-2-yl)carbamate ester
[0204]
[0205] Step 1 Preparation of 8-bromo-5-(4-tert-butylphenyl)-5H-benzo[e]imidazo[1,2-c][1,3]oxazine
[0206]
[0207] Using the compound 5-bromo-2-(1H-imidazol-2-yl)phenol and 1-(dibromomethyl)-4-tert-butylbenzene prepared in step 1 of embodiment 1 as raw materials, according to step 1 of embodiment 2 to obtain the title compound.
[0208] MS(ESI):[M+H] + =383.
[0209] Step 2 of 8-(1-ethoxyvinyl)-5-(4-tert-butylphenyl)-5H-benzo[e]imidazo[1,2-c][1,3]oxazine preparation
[0210]
[0211] The compound 8-bromo-5-(4-tert-butylphenyl)-5H-benzo[e]imidazo[1,2-c][1,3]oxazine obtained in step 1 is used as raw material, according to The title compound was prepared by the method in Step 3 of Ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com