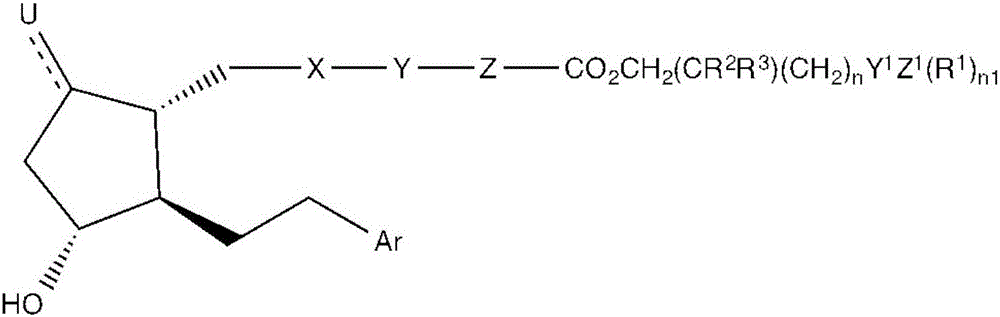Reduced central corneal thickening by use of hydrophilic ester prodrugs of beta-chlorocyclopentanes
A technology of alkyl and hydroxyalkyl, applied in the field of amino-oxoalkyl ester prodrugs, can solve the problems of insufficient solubility, overesterification, entering into aqueous humor and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
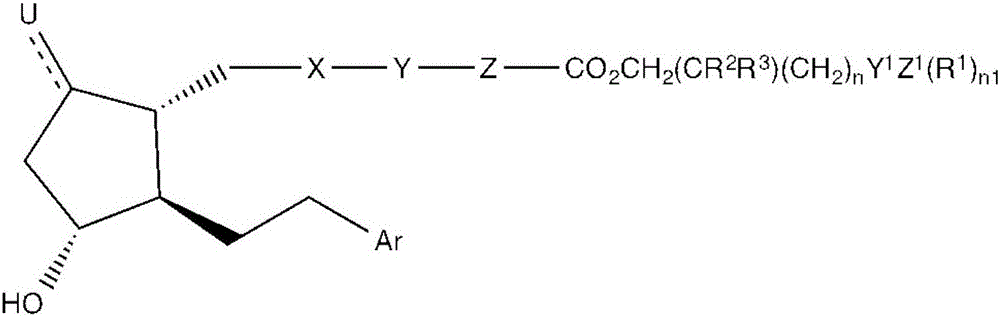
[0075] 1) A compound having the formula:
[0076]
[0077] in,
[0078] X is CH 2 CH 2 , cis-CH=CH, inner arylene, CH 2 S, CH 2 O or alkyne,
[0079] Y is C 1 –C 3 Alkyl, O, S, single bond, double bond or thiophene,
[0080] Z is an inner arylene group, C 1 –C 3 Alkyl, SCH 2 , OCH 2 , single or double bond,
[0081] U is O, Cl, H-H, H-OH, H-Cl, H-F or H-CN, and wherein the dashed bond represents a single or double bond,
[0082] R 1 independently selected from the group consisting of: H, C 1 -C 6 Alkyl and C 1 -C 6 Hydroxyalkyl, -OH and
[0083] R 2 independently selected from the group consisting of: H, C 1 –C 6 Alkyl, C 1 –C 6 Hydroxyalkyl and –OH,
[0084] R 3 independently H, C 1 –C 6 Alkyl and C 1 –C 6 Hydroxyalkyl, -OH or R,
[0085] n is 0-8,
[0086] Y 1 is C=O or a single bond,
[0087] Z 1 is N, NH, NH2, CH2 or -OH
[0088] n1 is 0-2,
[0089] R is H, alkyl and CH 2 Oh,
[0090] Ar is monosubstituted, disubstituted or trisub...
Embodiment 1
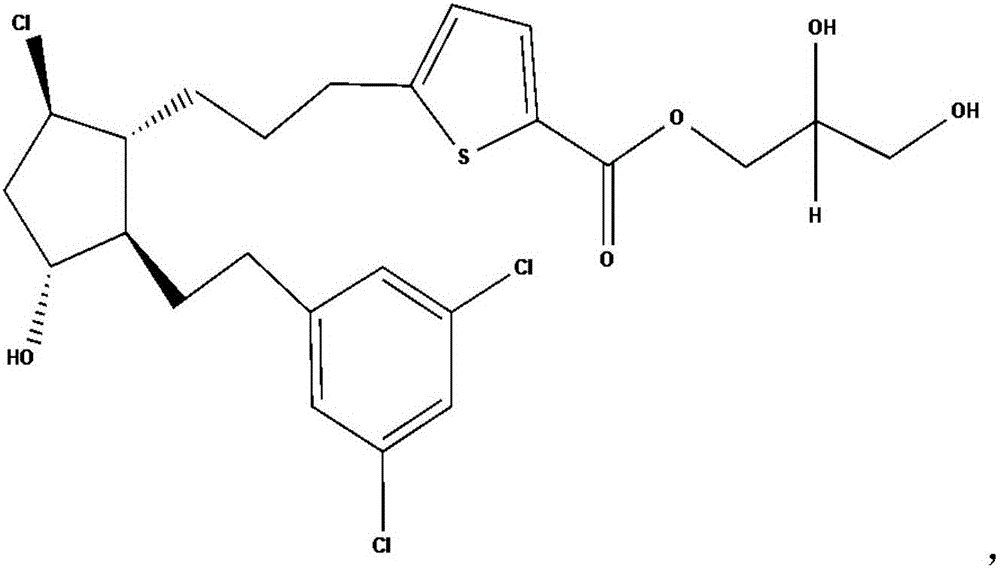
[0280] Example 1. (R)-2,3-dihydroxypropyl 5-(3-((1R,2R,3R,5R)-5-chloro-2-(3,5-dichlorophenethyl)- Synthesis of 3-hydroxycyclopentyl)propyl)thiophene-2-carboxylate (compound 3).
[0281] An exemplary synthesis of compound 3 is provided in Scheme 1 below.
[0282] ((R)-2,2-Dimethyl-1,3-dioxolan-4-yl)methyl 5-(3-((1R,2R,3R,5R)-5-chloro-2- (3,5-Dichlorophenethyl)-3-hydroxycyclopentyl)propyl)thiophene-2-carboxylate (Compound 2).
[0283]
[0284] (R)-(-)-2,2-Dimethyl-1,3-dioxolane-4-methanol (572.2 mg, 4.33 mmol) was added to the carboxylic acid of compound 1 (200 mg, 0.433mmol), 4-(dimethylamino)pyridine (55.3mg, 0.453mmol) and N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (91.3mg, 0.476 mmol) in DMF (3.0 mL). After stirring for 16 hours, the reaction solution was diluted with EtOAc and washed with 1N HCl, saturated aqueous NaHCO 3 , and then washed with brine. The organic portion was dried (MgSO 4 ), filtered and concentrated in vacuo. The residue was pu...
Embodiment 2
[0287] Example 2. ((S)-2,2-Dimethyl-1,3-dioxolan-4-yl)-methyl 5-(3-((1R,2R,3R,5R)-5 -Synthesis of chloro-2-(3,5-dichlorophenethyl)-3-hydroxycyclopentyl)propyl)thiophene-2-carboxylate (compound 4).
[0288]
[0289] According to the procedure described above for the preparation of compound 2, using 100 mg (0.216 mmol) of the carboxylic acid of compound 1 and 42.8 mg (0.324 mmol) of (S)-(+)-2,2-dimethyl-1, 3-Dioxolane-4-methanol afforded 72.3 mg (58%) of acetonide-protected ester compound 4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com