Application of 3-acetyl-11-keto-beta-boswellic acid to preparation of drug for treating periprosthetical osteolysis
A technology of periprosthetic bone and acetylation, which is applied in the direction of drug combination, bone disease, pharmaceutical formulation, etc., to achieve the effect of reducing the degree of osteolysis, increasing bone volume fraction, and inhibiting activation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
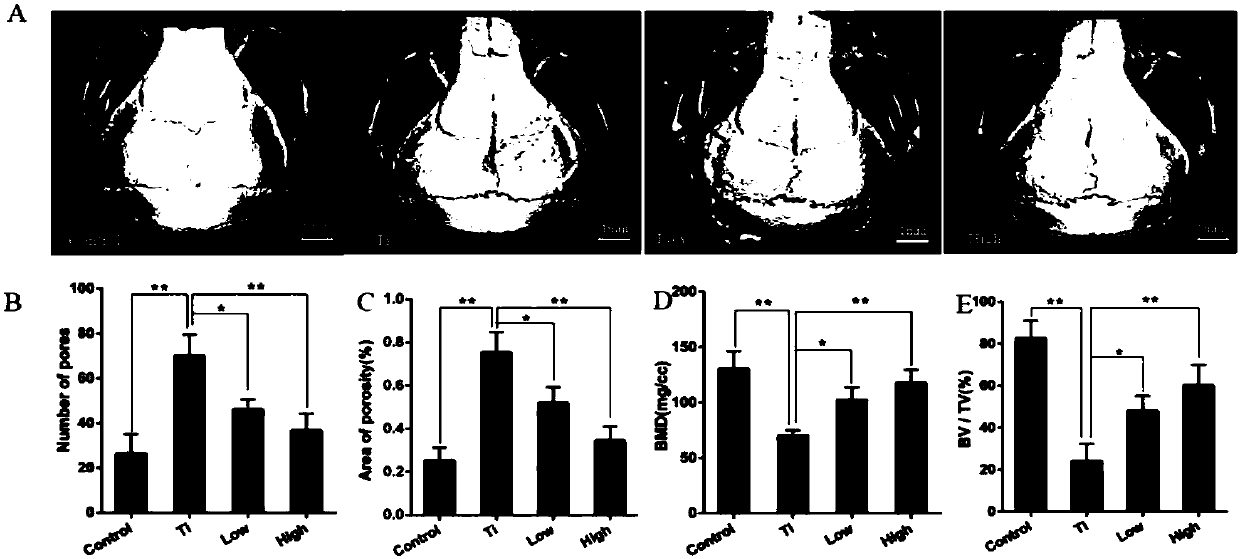
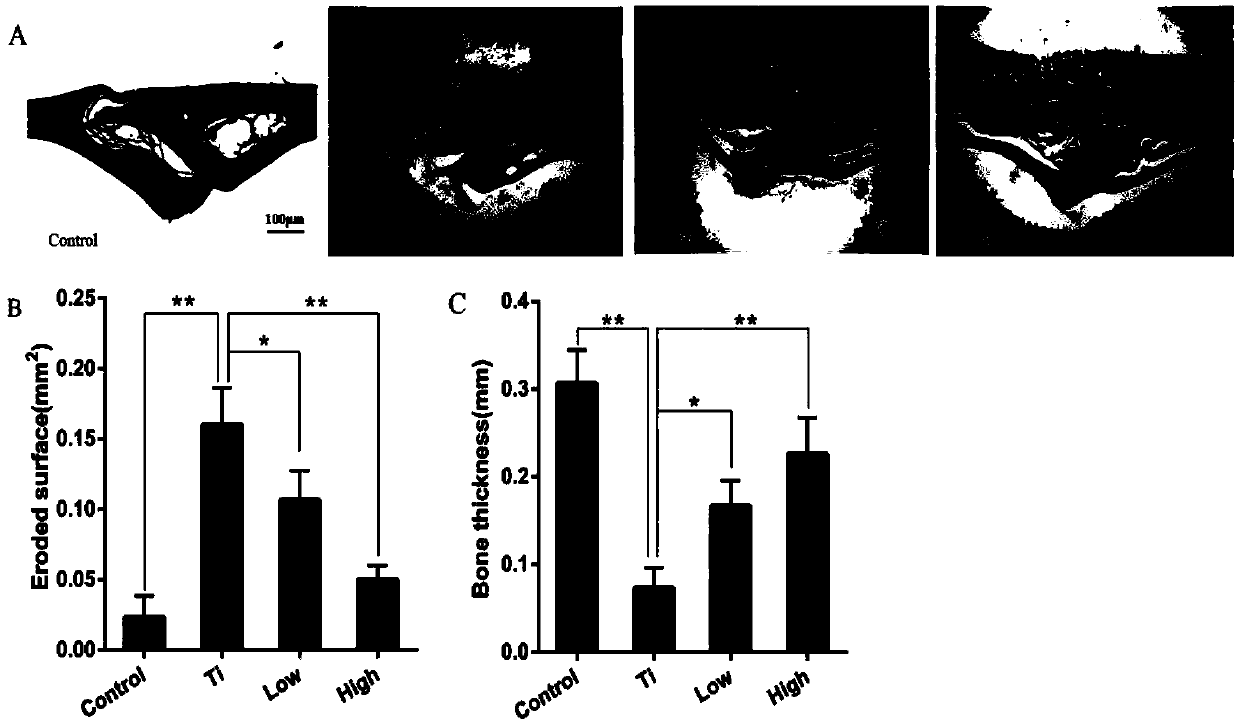
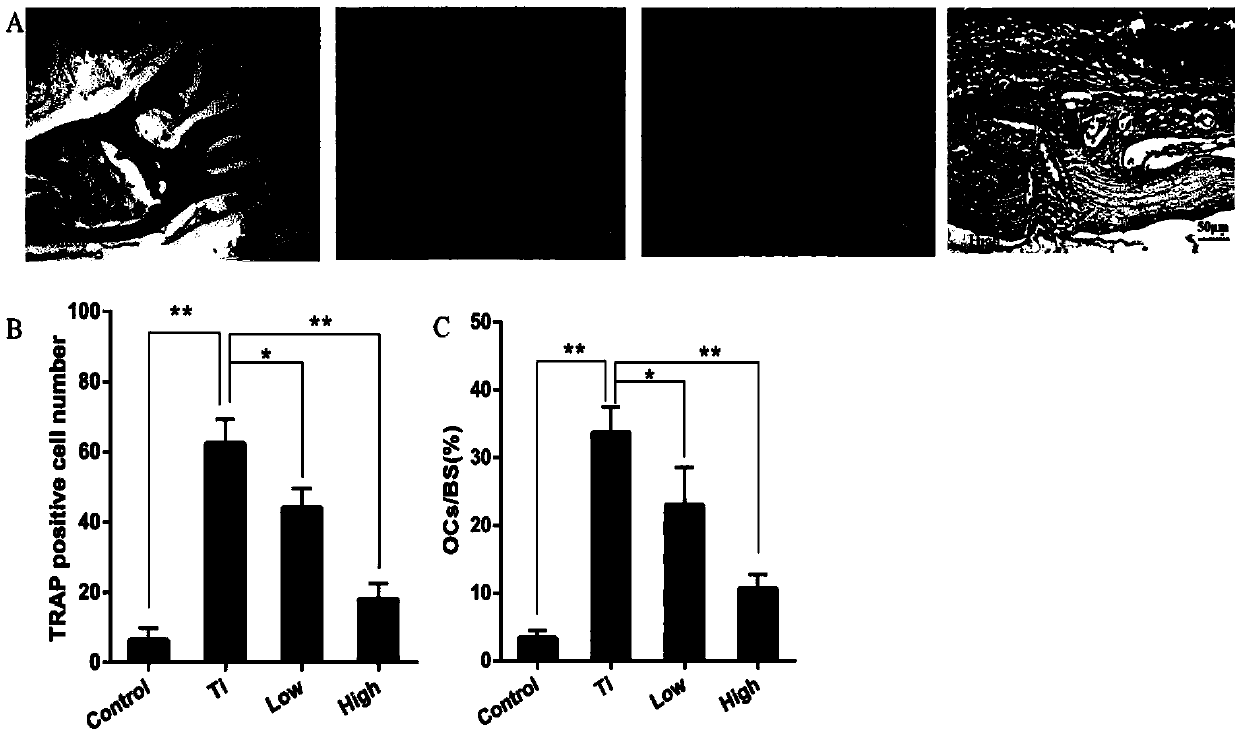
[0035] The establishment of embodiment 1 mouse skull osteolysis model
[0036] (1) Animal grouping
[0037] A total of 56 male C57BL / J6 mice were divided into 4 groups by random number table method: blank control group (Control), Ti particle group (Ti), AKBA low-dose treatment group (L-AKBA) and AKBA high-dose treatment group (H-AKBA), 14 mice in each group; each mouse was subcutaneously injected with 4 mg / kg of 6-chloro-α-methylcarbazole-2-acetic acid.
[0038] (2) Establishment of osteolysis model
[0039] The above four groups of mice were anesthetized with 4% chloral hydrate:
[0040] Blank control group (Control) only received surgery. A sagittal incision of about 1 cm was made in the middle of the skull of the mice to expose the periosteum of 1 cm × 1 cm, and then sutured. After the operation, 0.1 mL of sterile PBS solution was injected intraperitoneally into the mice every day , 6 days / week for 2 weeks;
[0041] In the Ti particle group (Ti), 20 mg of Ti particles w...
Embodiment 2
[0050] Example 2 Blood routine and blood biochemical index detection
[0051] The blood samples stored in routine blood tubes and biochemical tubes were taken out, and every 7 tubes of blood samples were taken as a group, and white blood cells (WBC), hemoglobin (Hb) and platelets (PLT) were detected on the automatic hematology analyzer respectively; Aspartate aminotransferase (AST), alanine aminotransferase (ALT), blood urea nitrogen (BUN) and serum creatinine (Cr) were detected on the analyzer. Statistical analysis was performed to evaluate the liver and kidney toxicity of mice after intervention with AKBA.
[0052] The results of blood routine showed that after 2 weeks of treatment with AKBA, the number of hemoglobin, white blood cells and platelets of the mice had no significant changes compared with those of the Ti particle group, and the difference was not statistically significant (p>0.05); Amino acid transaminase, aspartate transaminase, blood urea nitrogen and blood c...
Embodiment 3A
[0055] Example 3 AKBA inhibits the expression of inflammatory factors in the mouse skull in vitro culture system
[0056] The ELISA method was used to detect the contents of TNF-α, IL-1β and IL-6 in the culture supernatant of mouse skull in vitro, and the specific operation steps were as follows:
[0057] (1) Preparation of standard products: According to the ELISA kit instructions, dilute the standard products with the standard product diluent, and place them at room temperature for 10 minutes;
[0058] (2) There are 10 standard wells on the ELISA plate. Add 50 μL of the standard and the sample to be tested in each well, add 50 μL of the standard to one well as a blank well, and repeat the measurement 3 times for each sample;
[0059] (3) Add 50 μL of enzyme labeling solution to each well, mix the plate well, seal it with a membrane, and incubate in a 37°C incubator for 30 minutes;
[0060] (4) Discard the reaction solution, add 100 μL of washing solution to fully wash the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com