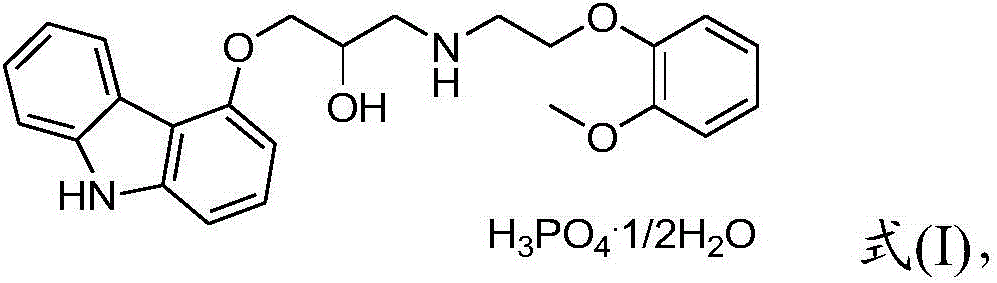
Preparation method of carvedilol phosphate hemihydrate
A technology of carvedilol phosphate and phosphoric acid, applied in the direction of organic chemistry, etc., can solve the problems affecting product purity and high dimer content, and achieve the effects of high purity and yield, simple synthesis method and high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
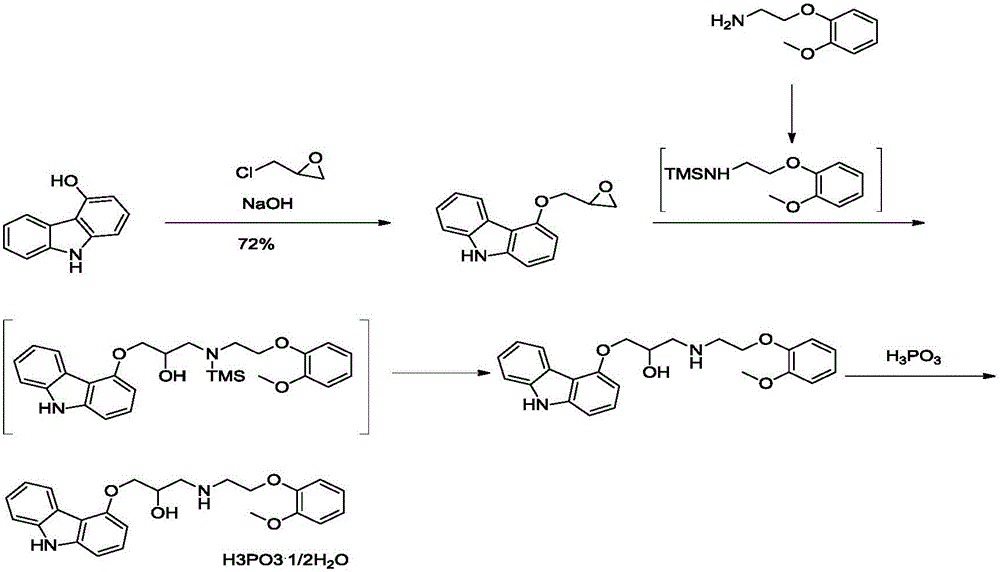
[0032] The invention provides a kind of preparation method of carvedilol phosphate, comprising:
[0033] 1) Mixing and reacting a compound having a structure of formula (II) with 2-(2-methoxyphenoxy)ethylamine to obtain a reaction solution containing a compound having a structure of formula (III);
[0034]
[0035] 2) adding phosphoric acid to the reaction solution obtained in step 1) to obtain 2-(2-methoxyphenoxy)ethylamine phosphate and filtrate,
[0036] The molar ratio of the 2-(2-methoxyphenoxy)ethylamine to the phosphoric acid is 1:(0.70~0.90);
[0037] 3) adding phosphoric acid to the filtrate obtained in step 2) to obtain carvedilol phosphate.
[0038] According to the present invention, the present invention mixes the compound having the structure of formula (II) with 2-(2-methoxyphenoxy)ethylamine to obtain a reaction solution containing the compound of formula (III); wherein, the The molar ratio of the compound having the structure of formula (II) to the 2-(2-m...
Embodiment 1
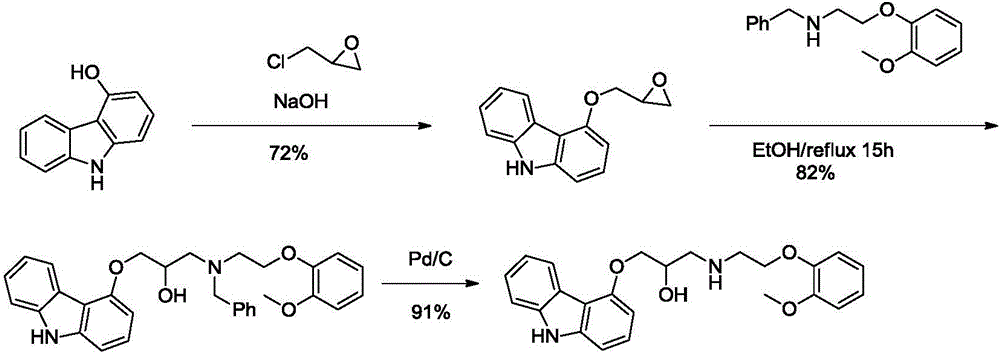
[0045] Preparation of 4-(2,3-epoxypropoxy)-carbazole (formula (II))
[0046] Add ethanol (300mL), water (300mL) and sodium hydroxide (13.2g, 0.33mol) to a 1000mL three-necked flask with mechanical stirring and stir to dissolve, then cool down to room temperature, 4-hydroxycarbazole (54.96g, 0.3mol) Add and stir for 60 minutes, add epichlorohydrin (30.53g, 0.33mol), stir and react at room temperature for 20 hours, filter, wash with 50% ethanol / water (100ml), and dry to obtain tan solid 4-(2,3 -Glycidyloxy)-carbazole (50.24g), the yield was 70%, and its purity was greater than 96% as detected by HPLC.
[0047] Preparation of carvedilol (formula (III))
[0048] Add absolute ethanol (800mL), 4-(2,3-epoxypropoxy)-carbazole (47.86g, 0.20mol) and 2-(2-methoxybenzene Oxy)ethylamine (167.09 g, 1.00 mol) was refluxed for 6 hours to obtain a reaction solution, which was then cooled and used directly in the next step.
[0049] Preparation of Carvedilol Phosphate
[0050] At room tempe...
Embodiment 2
[0054] The preparation of 4-(2,3-epoxypropoxy)-carbazole (formula (II)) is consistent with the examples.
[0055] Preparation of carvedilol (formula (III))
[0056] Add absolute ethanol (800mL), 4-(2,3-epoxypropoxy)-carbazole (47.86g, 0.20mol) and 2-(2-methoxybenzene Oxy)ethylamine (133.67 g, 0.80 mol) was refluxed for 6 hours to obtain a reaction liquid, which was then cooled and used directly in the next step.
[0057] Preparation of Carvedilol Phosphate
[0058] At room temperature, slowly add 80% phosphoric acid (88.17g, 0.72mol) dropwise (88.17g, 0.72mol) to the carvedilol ethanol solution obtained in the previous step at room temperature. Stir at room temperature for 1 hour, then filter, wash the filter cake with absolute ethanol (100 mL), and combine the filtrates for the next reaction. The filter cake is 2-(2-methoxyphenoxy)ethylamine phosphate.
[0059] Add water (45mL) to the obtained filtrate, slowly (3 hours) dropwise add 80% phosphoric acid (24.49g, 0.2mol) to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com