Liposome-based immunotherapy
A technology for liposomes and autoimmune diseases, applied in the medical field, can solve problems such as slowing down the progress, and achieve the effects of convenient uniformity, guaranteed uniformity, and easy access
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
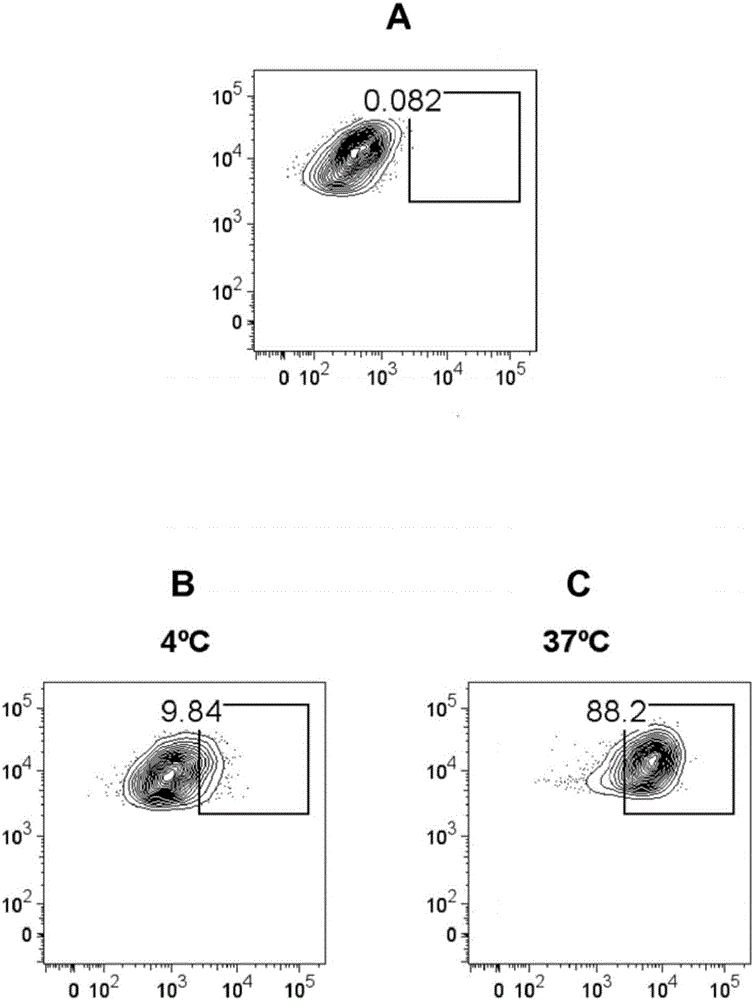
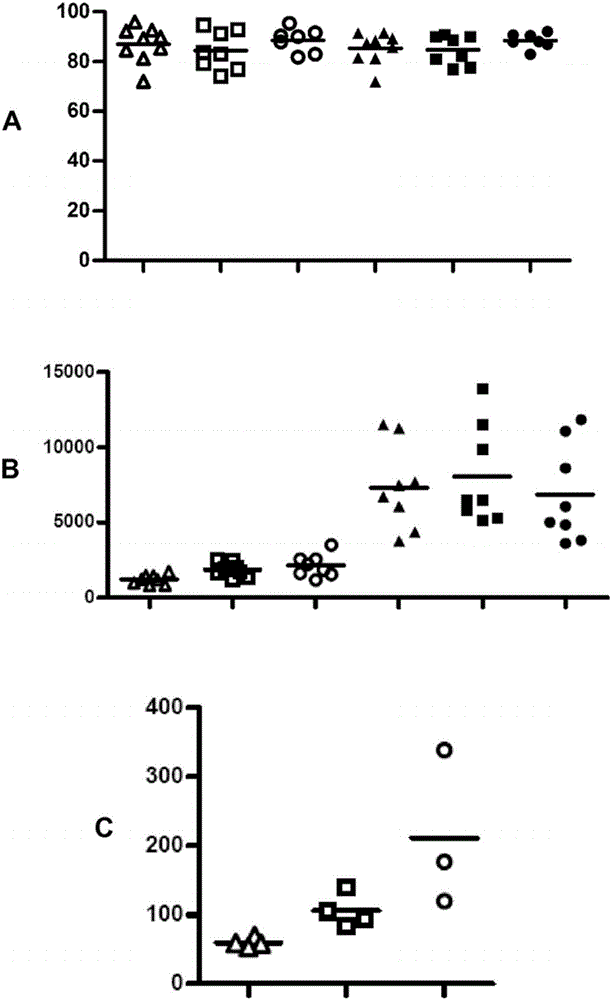
[0093] Materials and methods:
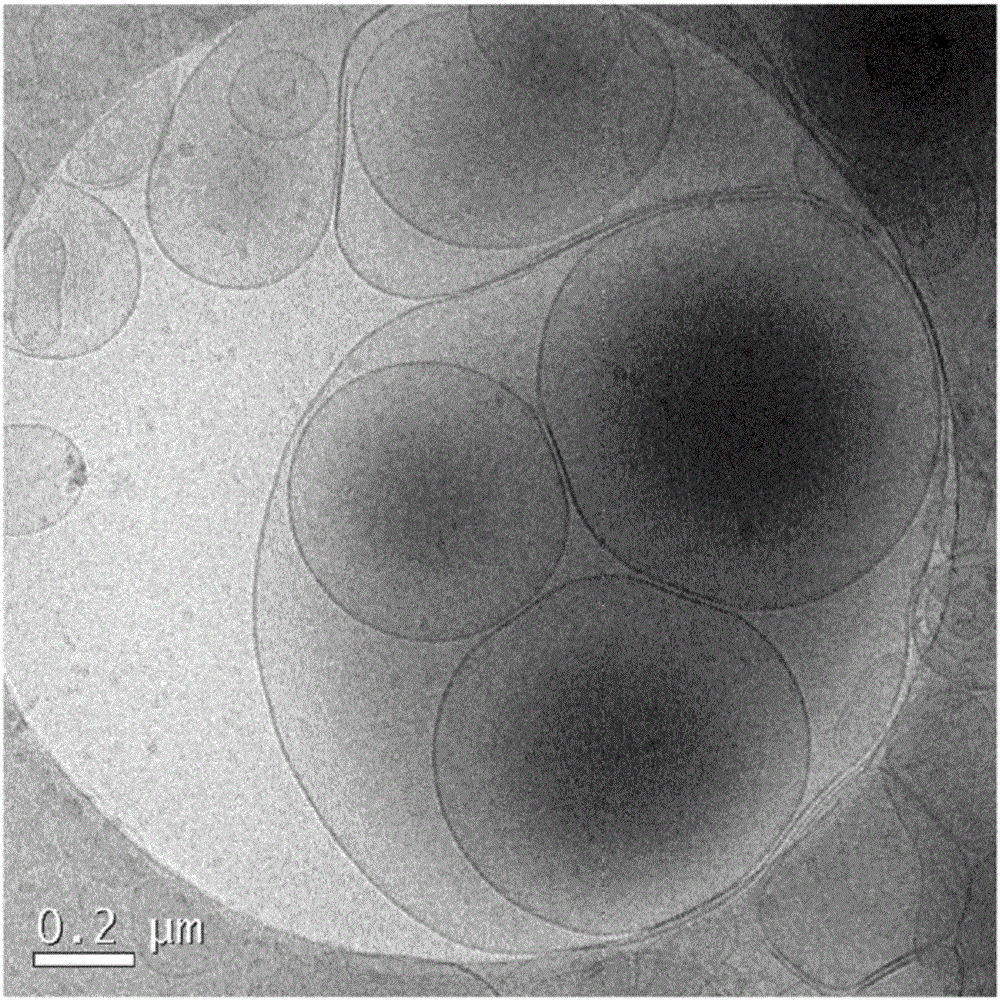
[0094] Preparation of liposomes
[0095] Phosphatidylserine (PS) and phosphatidylcholine (PC) were purchased from Lipoid, Steinhausen, Switzerland. Cholesterol (CHOL) was purchased from Sigma Aldrich, Saint Louis, USA. The lipid-conjugated fluorescent dye Oregon Green 488DHPE was purchased from Invitrogen, California, USA. Alexa Fluor 750 was obtained from Invitrogen in the form of its succinimide ester and was conjugated to lipid DOPE supplied by Avanti Polar Lipids (Alabaster, USA). Peptides of SEQ ID NO: 1 (GIVDQCCTSICSLYQLENYCN) and SEQ ID NO: 2 (FVKQHLCGSHLVEALYLVCGERGFFYTPMS) derived from insulin A chain and insulin B chain, respectively, were obtained from Genosphere Biotechnologies (Paris, France) with >95% purity and removal of trifluoro Acetic acid (tfa). The peptide of SEQ ID NO: 3 (YRSPFSRVVHLYRNGK) derived from myelin oligodendrocyte glycoprotein (MOG) was obtained from Institut de Recerca Biomèdica de Barcelona, IRBB, Barce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com