Compositions and methods for immunotherapy
a technology of immunotherapy and compositions, applied in the field of compositions and methods for immunotherapy, can solve the problems of significant limitations of dc immunotherapy, as many immunotherapies do, and achieve the effect of improving the immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Germline Humanized Variable Regions and Human Constant Region Sequences
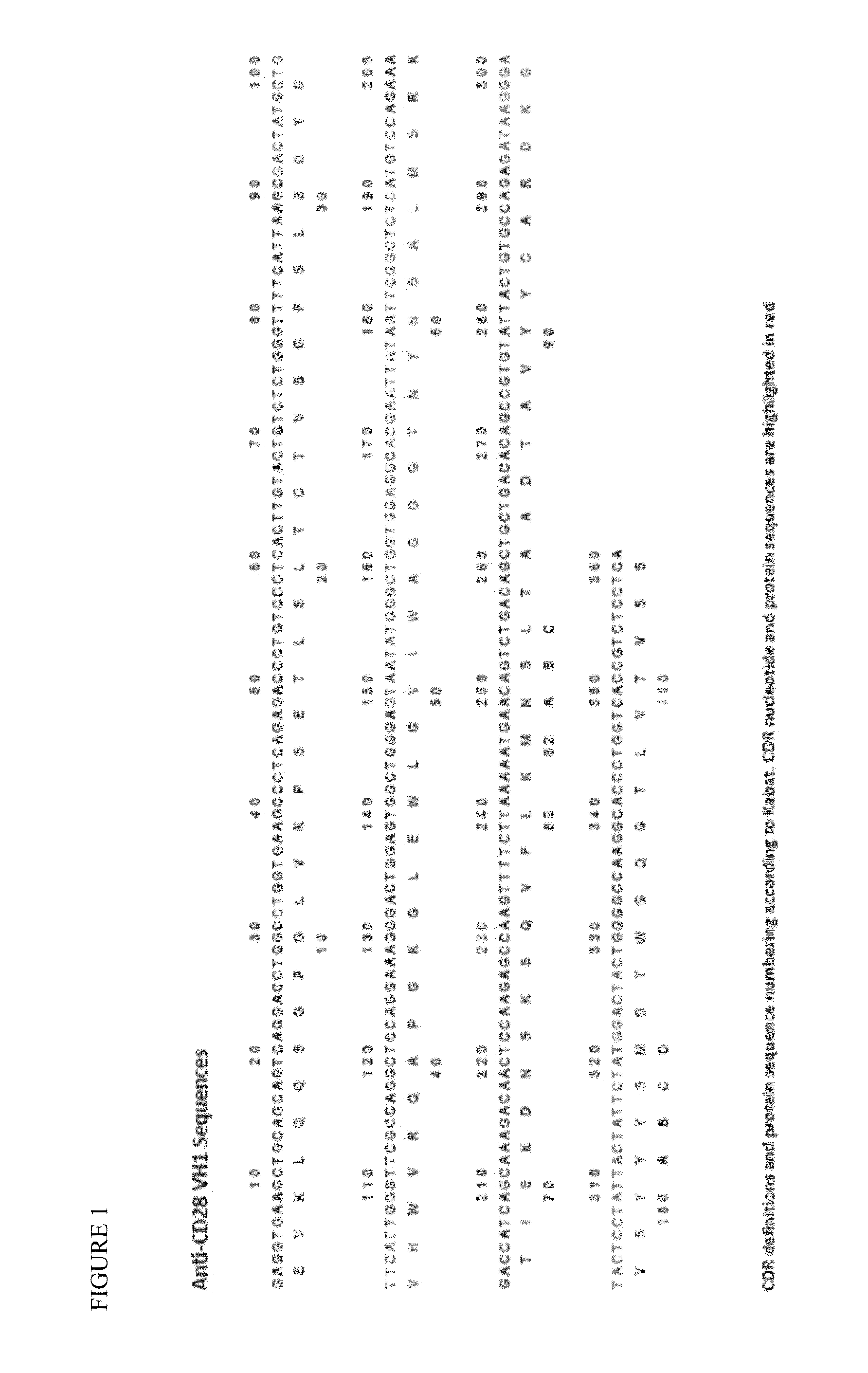
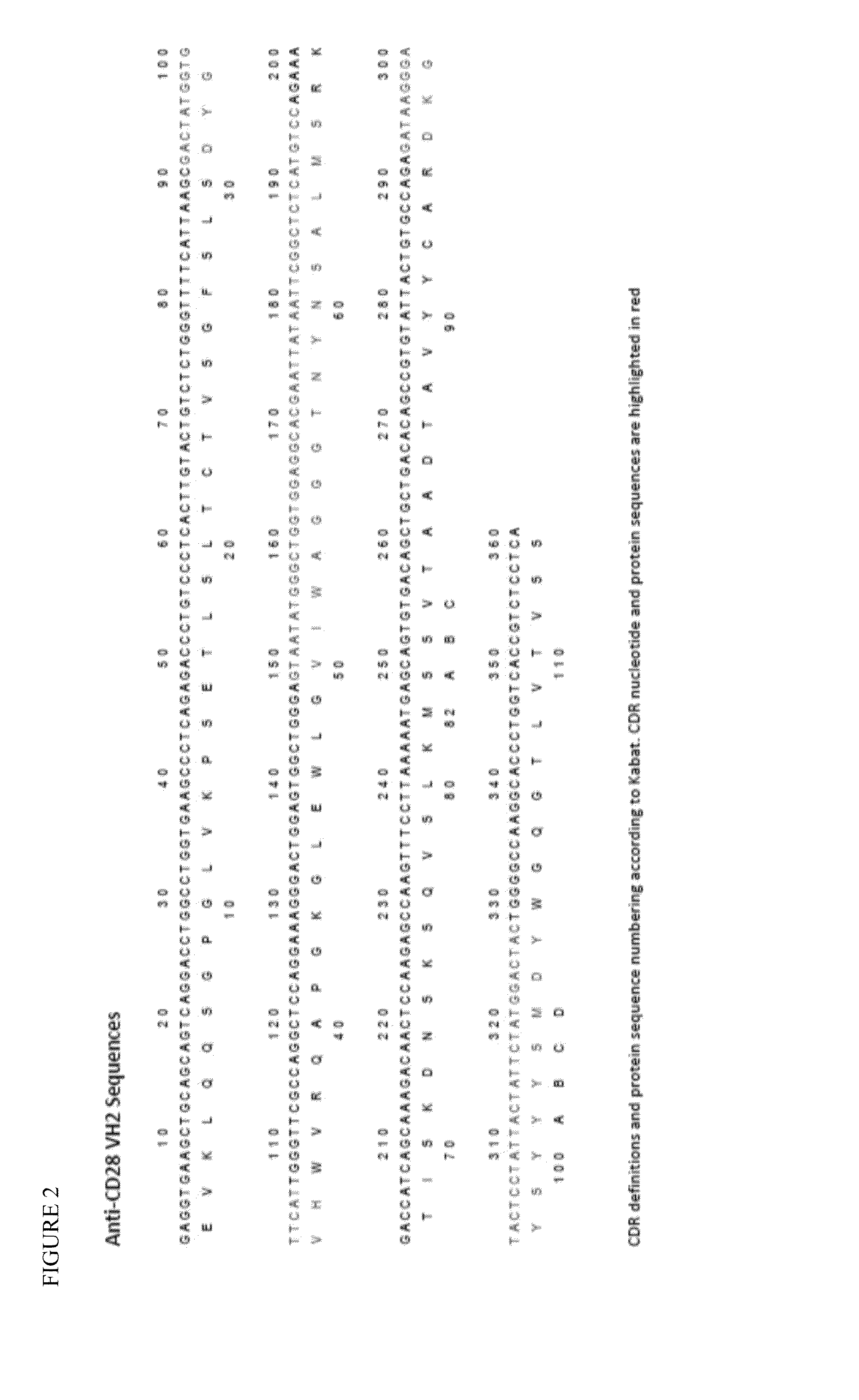
[0101]This Example demonstrates, inter alia, a design of sequences for germline humanized (CDR grafted) antibodies from a mouse anti-CD28 antibody template; a design of human constant region sequences including human IgG4 containing the S241P (Kabat numbering) hinge stabilizing mutation, the L248E (Kabat numbering) mutation to remove residual Fc gamma receptor binding and a Cys residue (S473C, Kabat numbering) suitable for coupling the antibody; a design of a variant germline humanized antibody V domain with potential non-binding to CD28; a design of a linker sequence for the fusion of HLA-A*02:01 to the N-terminus of the germline humanized antibodies that does not contain potential T cell epitopes.
[0102]The starting anti-CD28 antibody was the murine 9.3 monoclonal antibody (Tan et al. J. Exp. Med. 1993 177:165). Structural models of the 9.3 antibody V regions were produced using Swiss PDB and analyzed ...
example 2
Design of Linkers for Fusion of HLA-A*02:01 to Humanized Antibodies
[0112]Linkers for the fusion of HLA-A*02:01 (IMGT Accession No. HLA00005) to the N-terminus of humanized anti-CD28 antibodies were constructed and incorporated analysis by iTope™ and TCED™ to remove potential immunogenicity.
[0113]The iTope™ software predicts favorable interactions between amino acid side chains of a peptide and specific binding pockets (in particular pocket positions; p1, p4, p6, p7 and p9) within the open-ended binding grooves of 34 human MHC class II alleles. These alleles represent the most common HLA-DR alleles found world-wide with no weighting attributed to those found most prevalently in any particular ethnic population. Twenty of the alleles contain the ‘open’ p1 configuration and fourteen contain the ‘closed’ configuration where glycine at position 83 is replaced by a valine. The location of key binding residues is achieved by the in silico generation of 9 mer peptides that overlap by one am...
example 3
Codon Optimization of Sequences and Expression Cloning
[0119]Codons were optimized using GeneOptimizer®, and optimized sequences were cloned for expression as shown below.
[0120]Sequences were engineered with PmeI restriction sites, Kozak sequence, and signal peptide for expression in NS0 cells. Translation starts immediately downstream of the Kozak sequence.
[0121]The full translated amino acid sequence of the HLA-IgG4HC fusion is shown in FIG. 10.
[0122]The translated sequence of LC3 (VK3) is shown in FIG. 11.
[0123]The translated sequence for HC1 is shown in FIG. 12.
[0124]The translated sequence for HC2 is shown in FIG. 13.
[0125]Human β2 microglobulin was also expressed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com