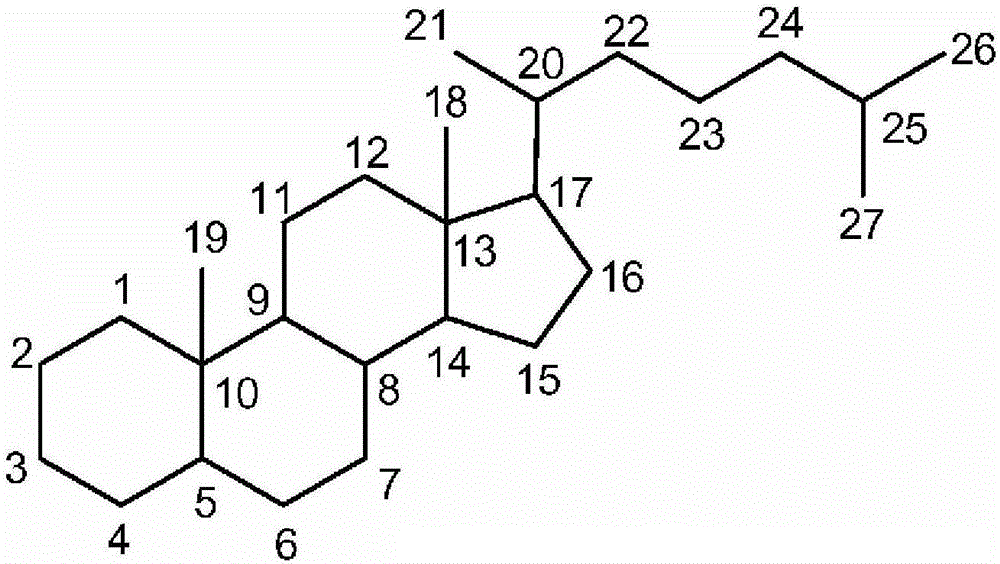
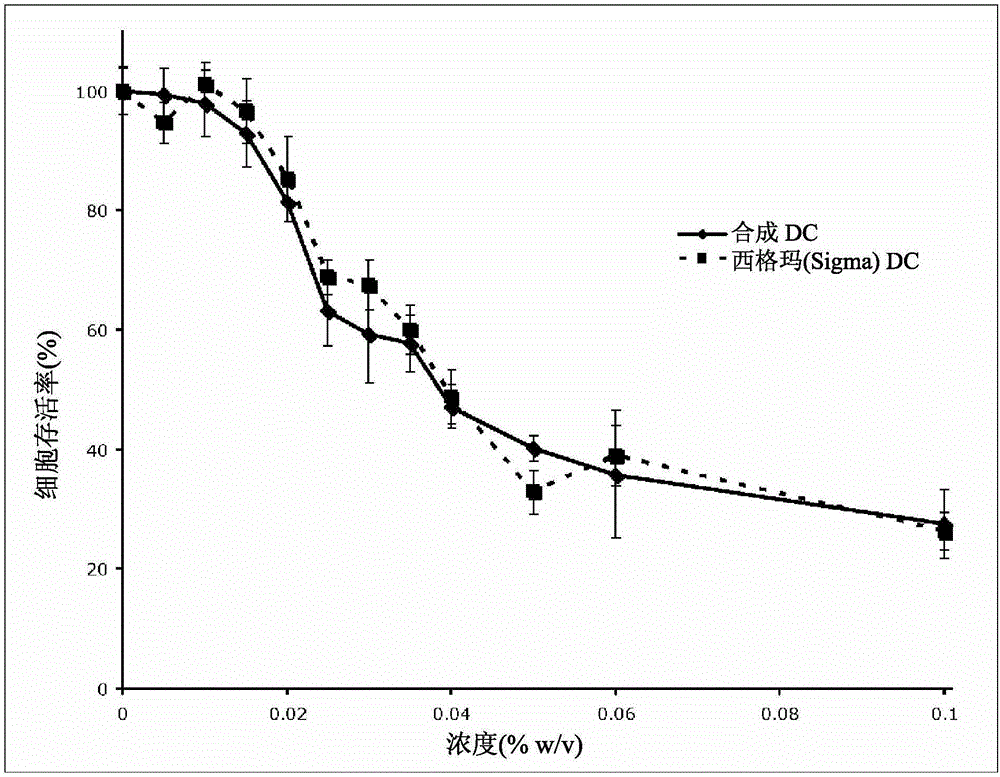
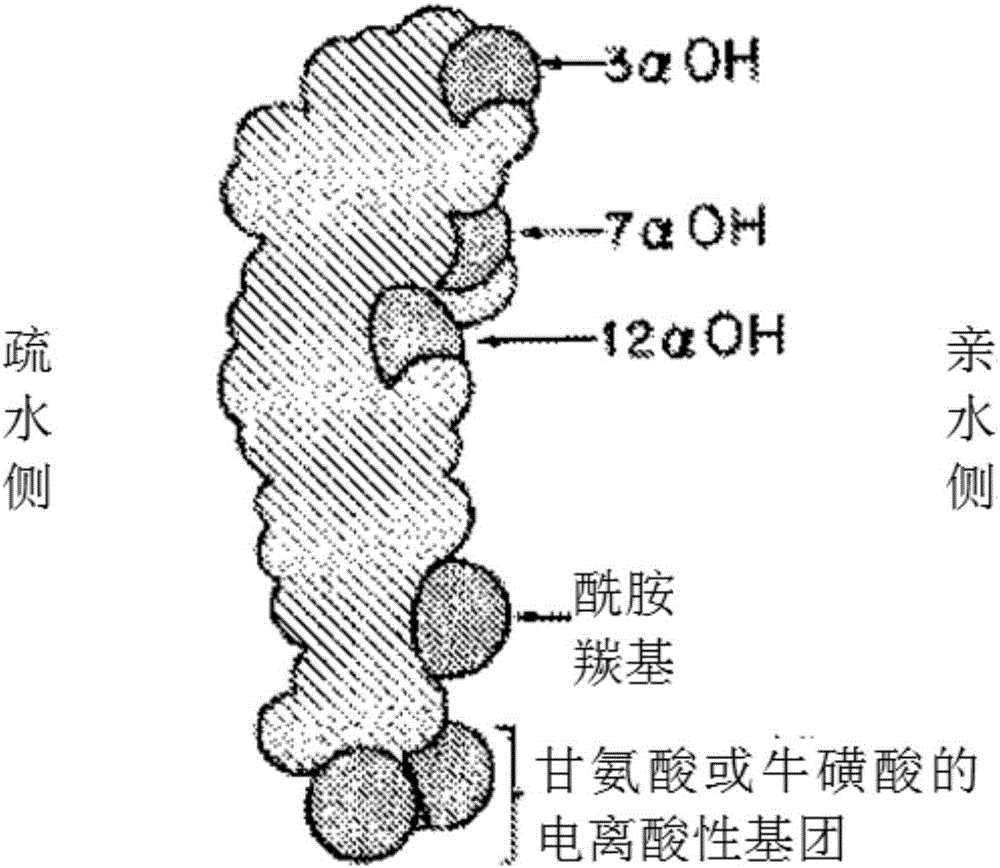
Synthetic bile acid composition, method, and preparation
A composition and compound technology, applied in the direction of drug combination, steroids, skin care preparations, etc., can solve the problem of undeveloped pharmaceutical grade composition of phytosterol-derived bile acids
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0027] definition
[0028] Throughout this disclosure, various publications, patents, and published patent specifications are hereby incorporated by reference by equivalents. The disclosures of these publications, patents, and published patent specifications are hereby incorporated by reference herein to more fully describe the state of the art to which this invention pertains.
[0029] Certain terms used herein have the meanings defined below. The singular forms "a, an" and "the" used in the specification and claims include both the singular and the plural unless the context clearly dictates otherwise.
[0030] Unless otherwise indicated, all numbers expressing quantities of ingredients, reaction conditions, etc. used in the specification and claims are to be understood in all instances as being modified by the term "about". Accordingly, unless indicated to the contrary, the numerical parameters set forth in the following specification and attached claims are approximations...
example
[0218] The various starting materials, intermediates and compounds of the preferred embodiments can be isolated and purified using conventional techniques such as precipitation, filtration, crystallization, evaporation, distillation, and chromatography, as appropriate. These compounds can be characterized using conventional methods such as melting point methods, mass spectrometry, nuclear magnetic resonance, and various other spectroscopic analyses.
[0219] Illustrative examples of steps for carrying out product synthesis in Scheme 1B of the first synthetic pathway are described in more detail below. Table 3 sets forth the abbreviations used in the Examples below and throughout the specification to express various compounds / moieties / devices / procedures / properties in exemplary reaction schemes and synthetic pathways.
[0220] table 3
[0221]
[0222]
[0223] General Procedures: Oxygen sensitive and moisture sensitive materials are handled in flame dried two-necked flas...
example 1
[0237] Preparation of androstane-3,11,17-trione (1.13)
[0238] 10% Pd / C (2.5 g, 5 wt%) was added to a solution of hydrocortisone (compound 1.12) (50.0 g, 138.12 mmol) in DMF (250 mL). The resulting slurry was hydrogenated in a Parr apparatus (50 psi) for 12h. After complete disappearance of the starting material as shown by TLC, the crude reaction mixture was filtered through a small plug of celite and the solvent was removed under vacuum. The crude product (48.0 g) was obtained in the form of a colorless solid.
[0239] NaBH 4 (2.1 g, 55.3 mmol) was added to a solution of EtOH (500 mL) and CH 2 Cl 2 (500 mL) in a solution of the above crude product (48.0 g, 131.86 mmol). After 1 h, acetone (50 mL) followed by water (150 mL) and NaIO were added sequentially 4 (70.5 g, 329.6 mmol). The mixture was stirred overnight at room temperature.
[0240] Distilled water (500 mL) was added and the mixture was extracted with ethyl acetate (3 x 250 mL). The ethyl acetate layer was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com