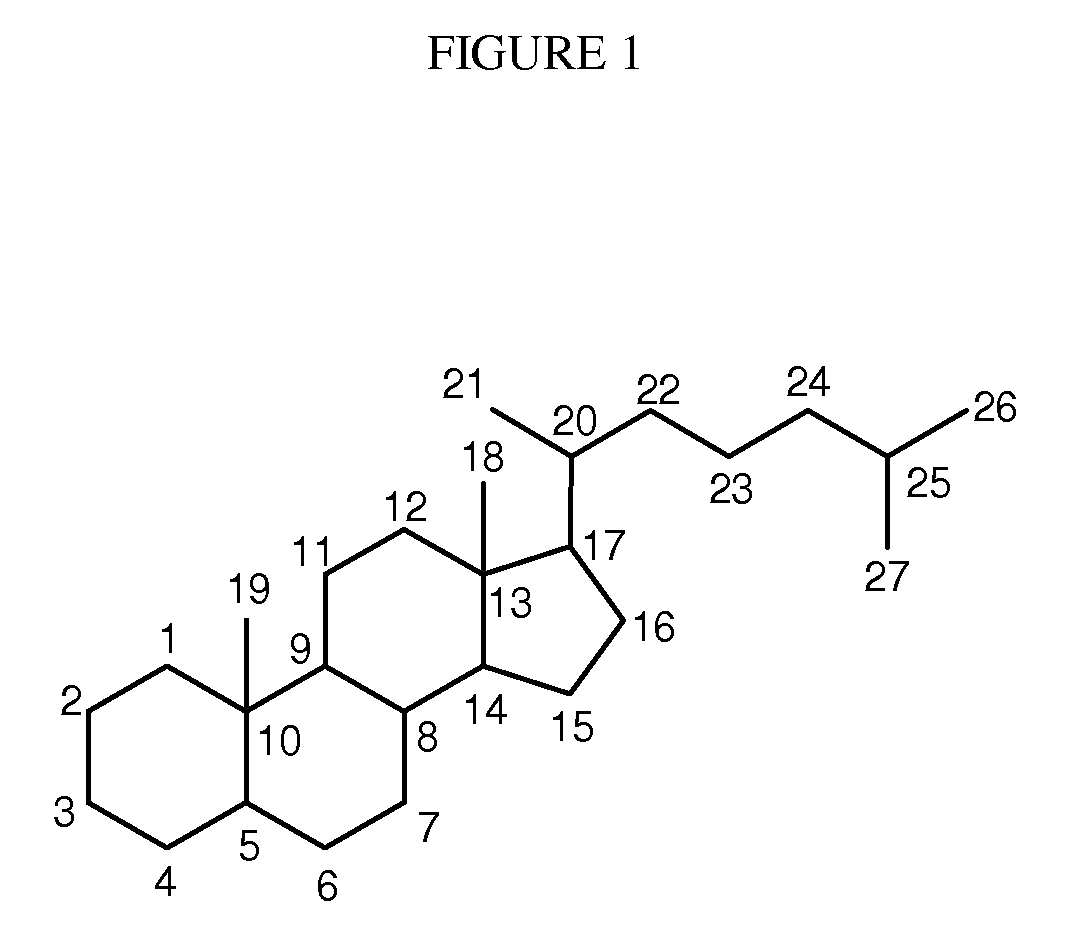
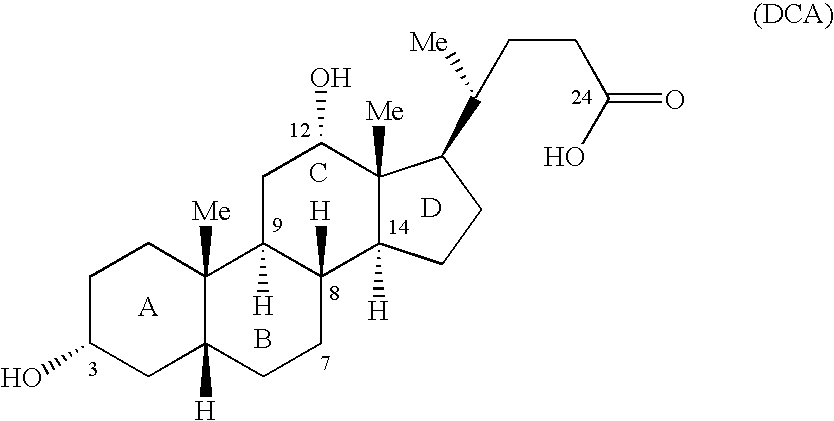
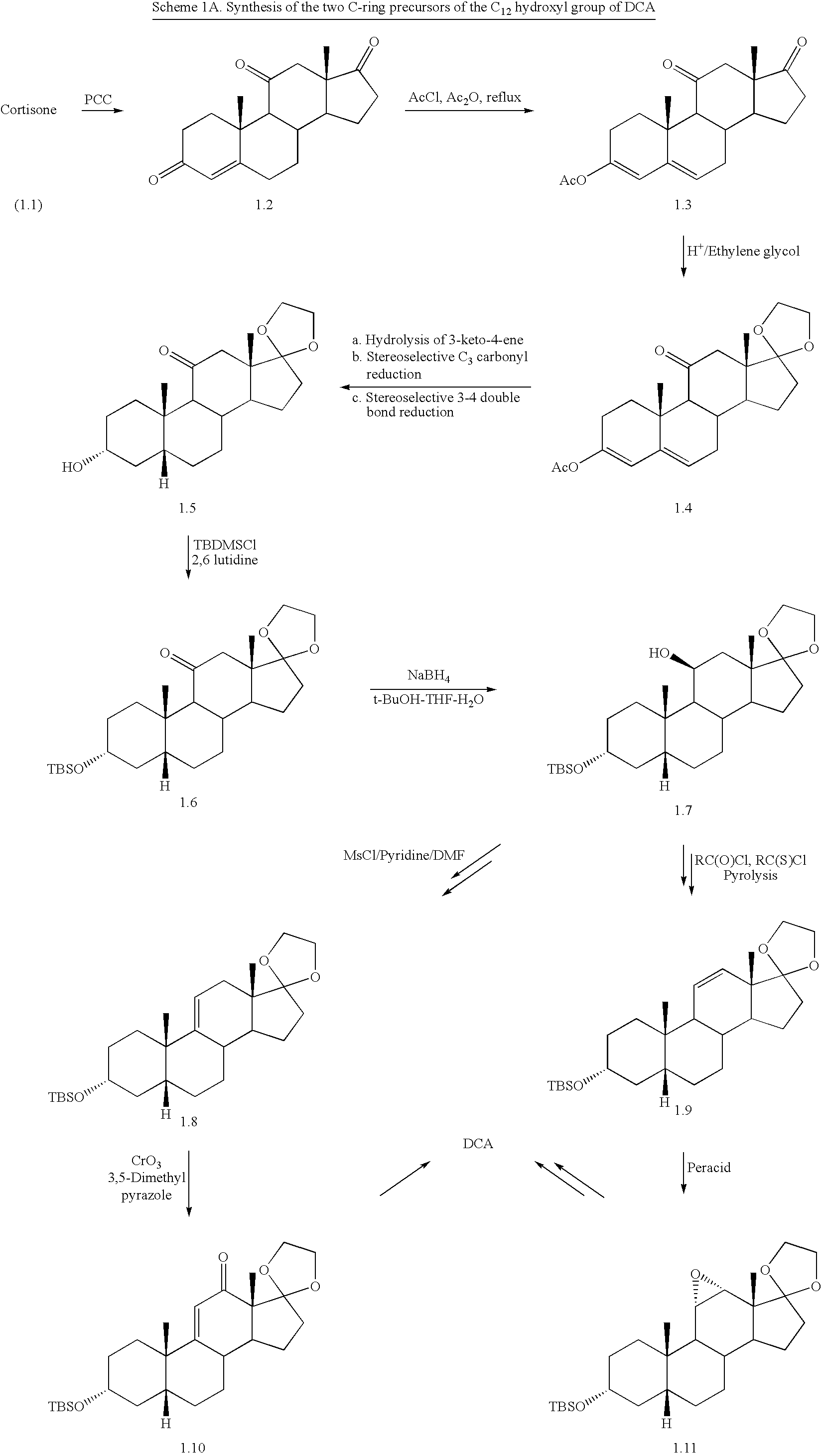
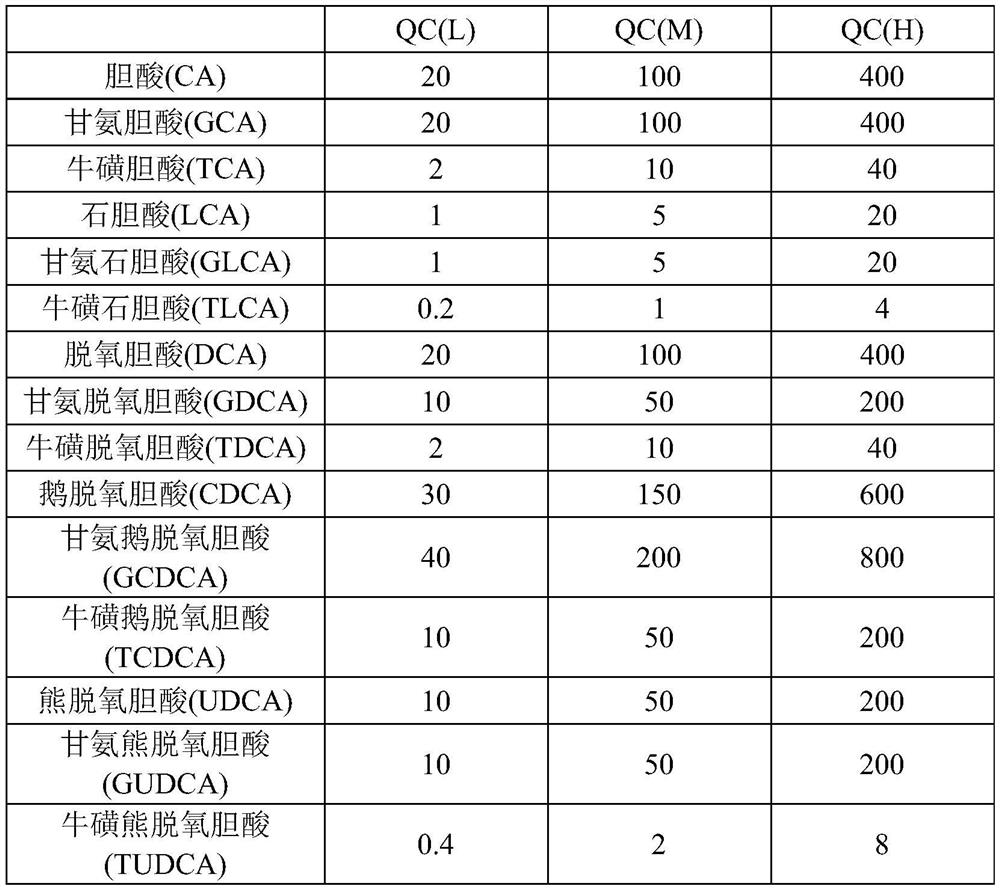
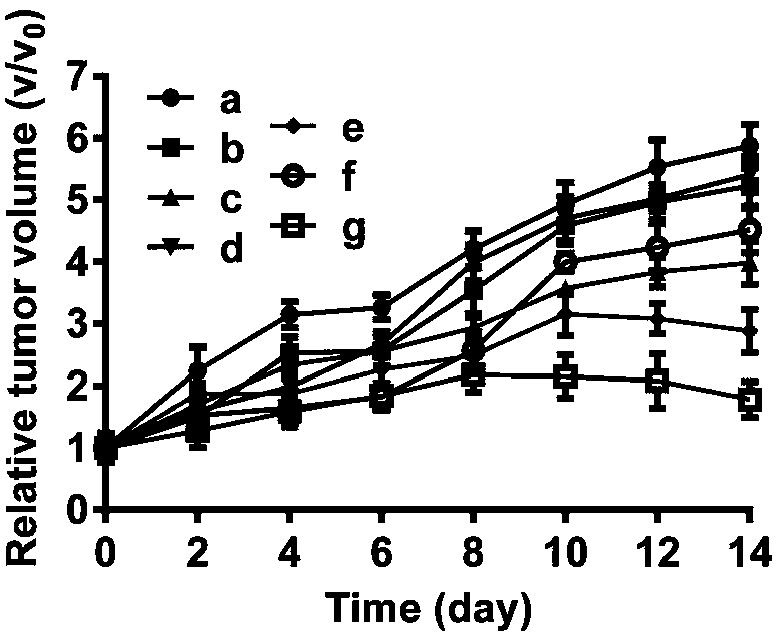
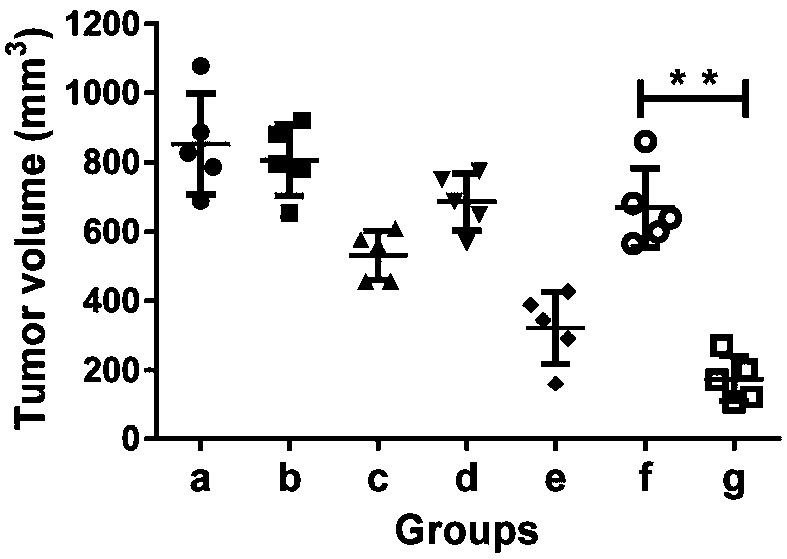
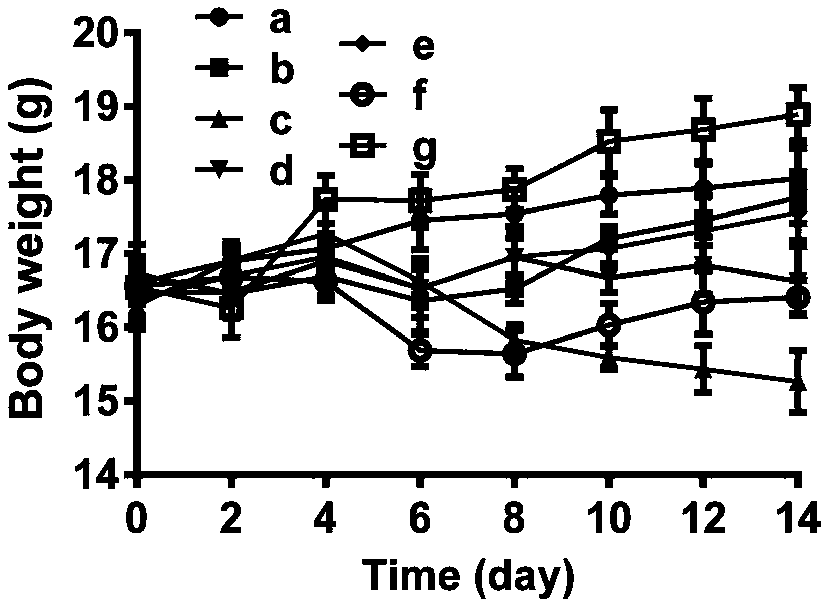
Patents
Literature
58 results about "Desoxycholic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Deoxycholic acid is used to help decrease the appearance of fat that hangs below the chin, sometimes called a double-chin. Deoxycholic acid has not been tested for safe use on other areas of the body. Deoxycholic acid may also be used for purposes not listed in this medication guide.
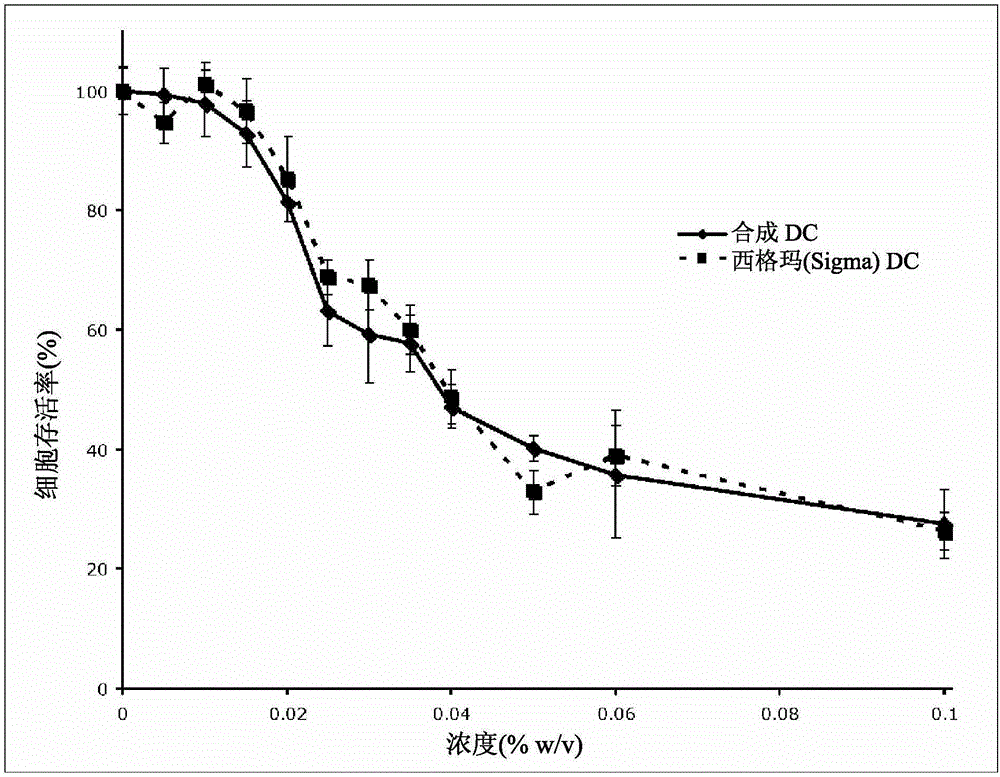
Synthetic bile acid compositions and methods
Bile acids and related compositions and methods of synthesis and use. More specifically, deoxycholic acid and related compositions, said compositions being free of all moieties of animal origin and free of pyrogenic moieties.
Owner:ALLERGAN SALES LLC
Amphipathic chitosan derivative and preparation method and application thereof
InactiveCN102241790ARich sourcesPreparation reaction conditions are mildOrganic active ingredientsGenetic material ingredientsChemical reactionClick chemistry
The invention discloses an amphipathic chitosan derivative PAMAM-Cs-DCA (Poly(amido amine)-chitosan-deoxycholic acid). The PAMAM-Cs-DCA is prepared by sequentially grafting a PAMAM unit and a deoxycholic acid unit on a main chain of chitosan by click chemical reaction and amidation reaction. The preparation method has mild reaction conditions, high efficiency and selectivity. The invention also discloses an application of the amphipathic chitosan derivative in preparing an anticancer drug carrier: the amphipathic chitosan derivative forms nanomicelle which takes the PAMAM unit and chitosan asa hydrophilic shell and takes the DCA unit as a hydrophobic core by self assembly in a water solution, wherein hydrophobic anticancer drugs can be coated in the core, and the shell can be compounded with pDNA (plasmid deoxyribonucleic acid) to realize co-transmission of the drugs and genes. Due to the unique molecular structure, the amphipathic chitosan derivative has potential application valuesin the fields of gene therapy, controlled release of drugs, tissue engineering and the like.
Owner:SUN YAT SEN UNIV
Compositions and methods for therapeutic use
A method and pharmaceutical composition for the enhancement of transfer of a therapeutic agent to a cell wherein the therapeutic agent is formulated in a buffer comprising a compound of Formula I:wherein:n is an integer from 2-8; X1 is a cholic acid group or deoxycholic acid group; and X2 and X3 are each independently selected from the group consisting of a cholic acid group, a deoxycholic acid group, and a saccharide group, wherein the saccharide group is selected from the group consisting of pentose monosaccharide groups, hexose monosaccharide groups, pentose-pentose disaccharide groups, hexose-hexose disaccharide groups, pentose-hexose disaccharide groups, and hexose-pentose disaccharide groups; and wherein at least one of X2 and X3 is a saccharide group.
Owner:MERCK SHARP & DOHME CORP
Kit for testing 3-sulfate glycochenodeoxycholic acids and glycochenodeoxycholic acids in blood
InactiveCN101995441AImprove the ability of separation and resolutionSuitable for early screeningComponent separationBiological testingInternal standardSulfate
The invention discloses a kit for testing 3-sulfate glycochenodeoxycholic acids and glycochenodeoxycholic acids in blood, which consists of internal standard isoleucine peptides, buffer solution ammonium acetates and acetonitrile. A method for testing the 3-sulfate glycochenodeoxycholic acids and glycochenodeoxycholic acids in blood by using the kit has the characteristics of low testing cost, good repeatability, high stability, speediness, and high efficiency; and a test on an actual sample by the kit only takes19 minutes, therefore, the kit is suitable for clinical application.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Method for preparing protamine-deoxycholic acid conjugate with heparin transfer function
InactiveCN103623421AInhibition of growth and proliferationImprove reprinting efficiencyPowder deliveryOrganic active ingredientsCross-linkNano composites
The invention belongs to the technical field of biological medicine, and particularly relates to a method for preparing a protamine-deoxycholic acid conjugate with a heparin transfer function. The amidogen of protamine and the carboxyl of deoxycholic acid form an amide bond through teh effect of a cross-linking agent to prepare an amphipathic conjugate, and then the amphipathic conjugate and heparin form a self-assembled aggregation in order to conveniently release the heparin in cells. The hydrophobic modification of the conjugate can enhance the self-aggregation stability of nano-composites; the cationic performance of the conjugate entraps the heparin, so that the distribution capability of the heparin in the cells is increased, and the heparin is prevented from, degradation caused by the effect of heparinase and further the transfer of the heparin into cancer cells and the release and biological effect of the heparin in the cells are realized.
Owner:JIANGNAN UNIV
Synthetic bile acid composition, method, and preparation
InactiveCN106083969AOrganic active ingredientsCosmetic preparationsSynthetic bile acidDesoxycholic acid
The invention relates to bile acids and related compositions and methods of synthesis and use. More specifically, deoxycholic acid and related compositions, said compositions being free of all moieties of animal origin and free of pyrogenic moieties.
Owner:艾尔健销售有限责任公司
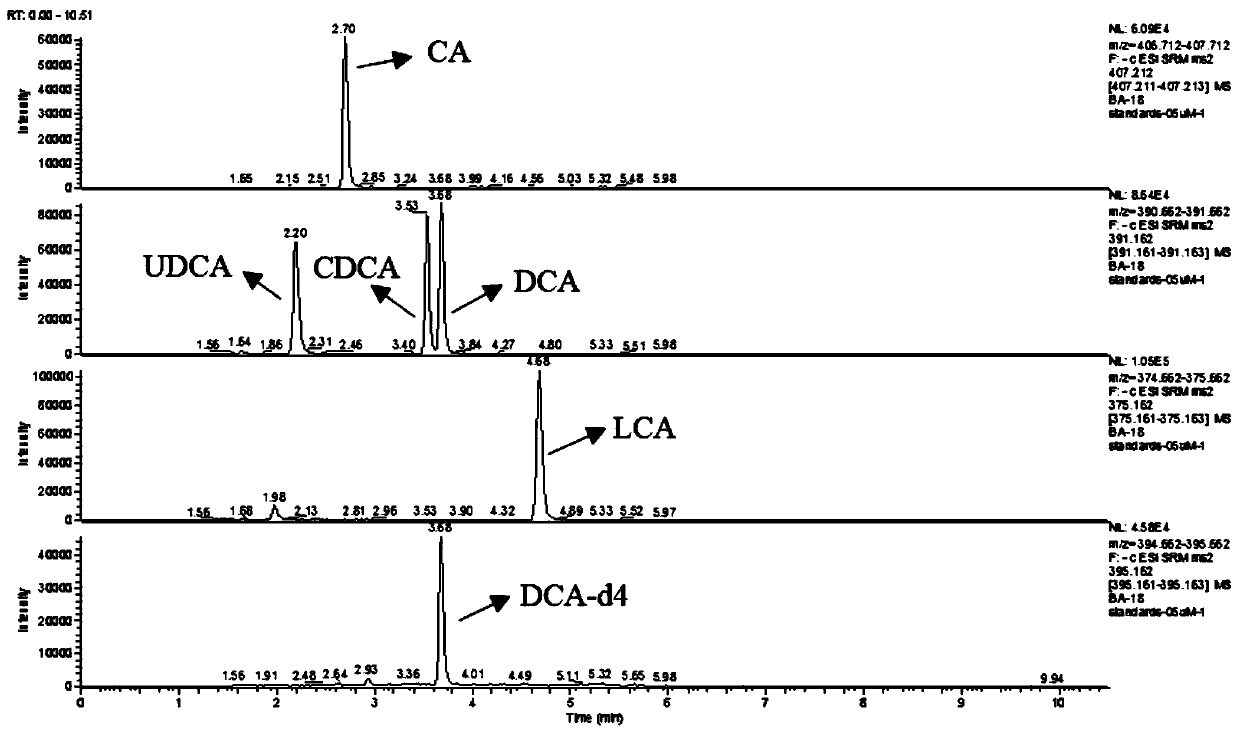
Mass spectrum kit for accurately determining concentration of bile acid in serum
InactiveCN111474288ASmall sample sizeHigh detection specificityComponent separationInternal standardMass analyzer
The invention discloses a mass spectrum kit for accurately determining the concentration of bile acid in serum. The mass spectrum kit is characterized by comprising a calibrator containing 15 kinds ofbile acid, an internal standard of deoxycholic acid-d4, glycodeoxycholic acid-d4 and taurodeoxycholic acid-d4, a quality control material of the 15 kinds of bile acid, an extracting solution, a mobile phase and a reconstitution fluid. The method has the advantages that complex purification steps are not needed, the required sample size is small, and the method can be matched with a liquid chromatograph tandem mass spectrometer for use and is applied to inspection of clinical samples. Meanwhile, the product can detect 15 bile acids with different structures at the same time, achieves a single-sample multi-index synchronous detection function, and has the characteristics of high accuracy, short detection time, low reagent consumption, convenience in operation and the like. In addition, themeasurement result can be directly traced back to the reference system, thereby guaranteeing the accuracy of the measurement result.
Owner:NINGBO MEDICAL SYSTEM BIOTECHNOLOGY CO LTD
Mesotherapy Cream
InactiveUS20080058287A1Promotes breakdown and leachingTo promote metabolismBiocidePhosphorous compound active ingredientsCholic acidBULK ACTIVE INGREDIENT
The present disclosure concerns a cream-based composition and method which reduces fat deposits upon topical application to the skin. The active ingredients of the composition include the active ingredients deoxycholic acid and phosphatidyl choline which are mixed together with a cream composition. The active ingredients may be combined with any suitable pharmaceutical vehicle to provide the fat-reducing composition claimed herein. The composition is topically applied to a skin area over a period of days or months in order to reduce fat deposits that have collected beneath the dermis.
Owner:NATURAL DESIGNS
Preparation method of novel amphiphilic polymer of deoxycholate-modified chitosan oligosaccharide
InactiveCN110354100AImprove stabilityMild reaction conditionsOrganic active ingredientsPharmaceutical non-active ingredientsDelivery systemPolymer
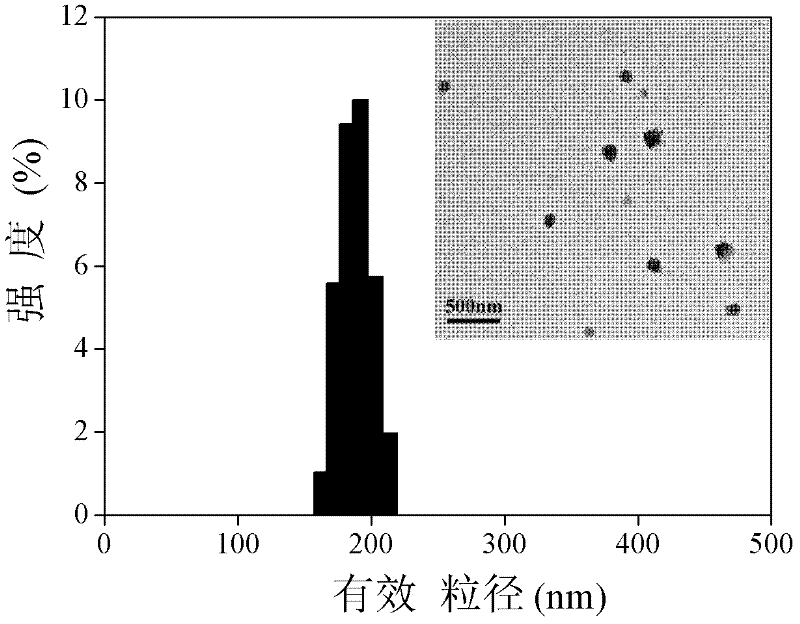
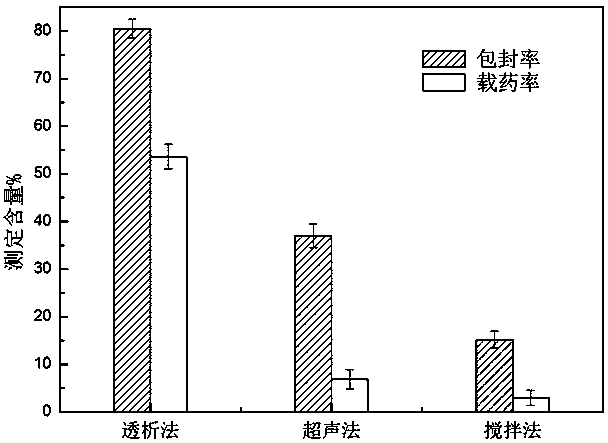
The invention discloses a preparation method of a novel amphiphilic polymer of deoxycholate-modified chitosan oligosaccharide. The preparation method is applied to the encapsulation of oleanolic acid,aims at improving the bioavailability and stability of the oleanolic acid, and comprises the following steps that 1, firstly, chitosan oligosaccharide and a deoxycholate solution are formed in an organic solvent; 2, the deoxycholate is grafted to the chitosan oligosaccharide to obtain the amphiphilic polymer of the deoxycholate-modified chitosan oligosaccharide; 3, the amphiphilic polymer is added into an organic solvent to form a deoxycholate-modified chitosan oligosaccharide dispersion solution; 4, an oleanolic acid solution is dropped into the deoxycholate-modified chitosan oligosaccharidesolution to form deoxycholate-chitosan oligosaccharide encapsulated oleanolic acid nanoparticles through self-assembly. The maximum encapsulation rate and drug loading rate of the obtained nanoparticles are 80.50% and 53.61% respectively, the particle diameter is 250 + / - 25 nanometers, the bioavailability of the oleanolic acid is significantly improved, the application of the physiological functions of the oleanolic acid in the medical field is enhanced, and a novel material is provided for an oral delivery system of hydrophobic drugs.
Owner:HARBIN INST OF TECH
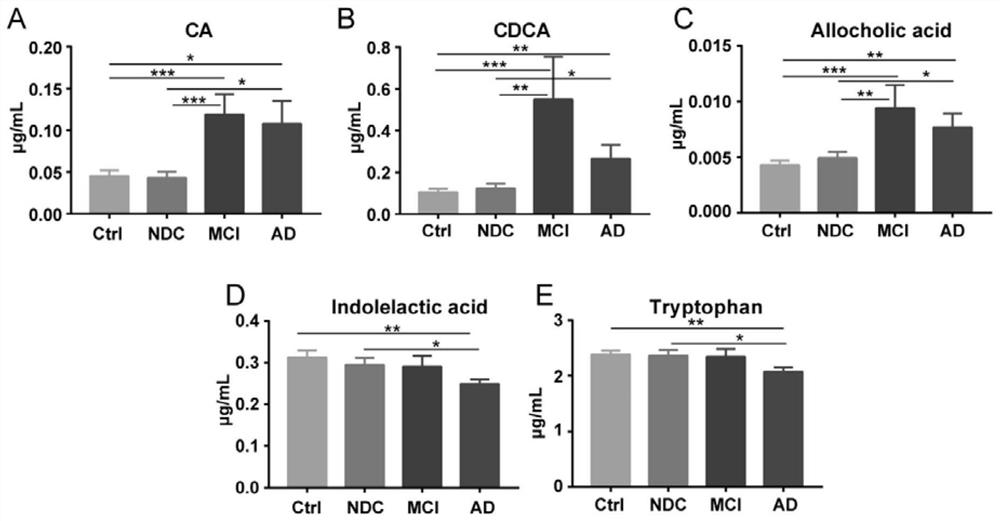
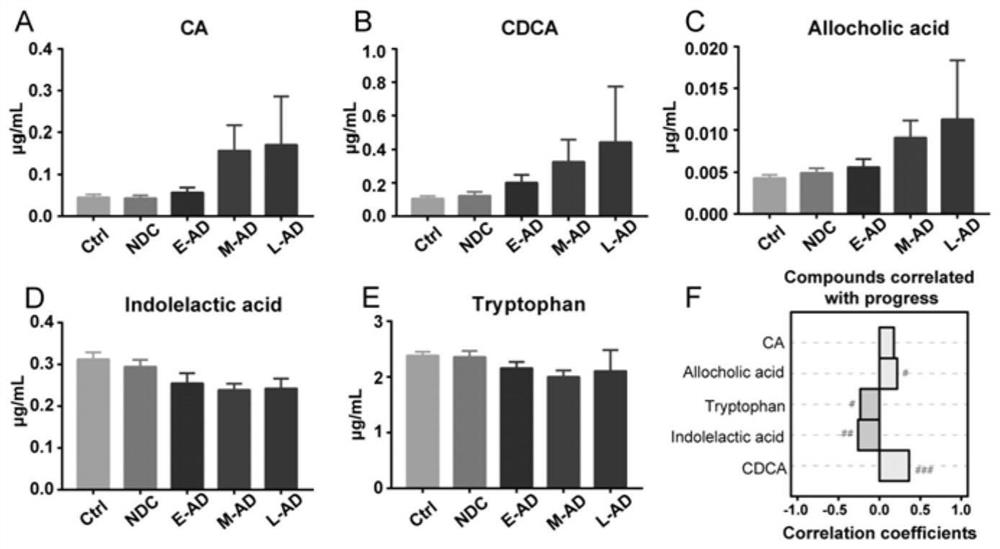
Use of combined serum metabolic marker in preparation of kit for diagnosing progress of hepatopathy, kit, and method using kit to screen serum metabolic markers
InactiveCN107656007AAlleviate the problem of difficult development monitoringRapid diagnosisComponent separationSerum igeMetabolite
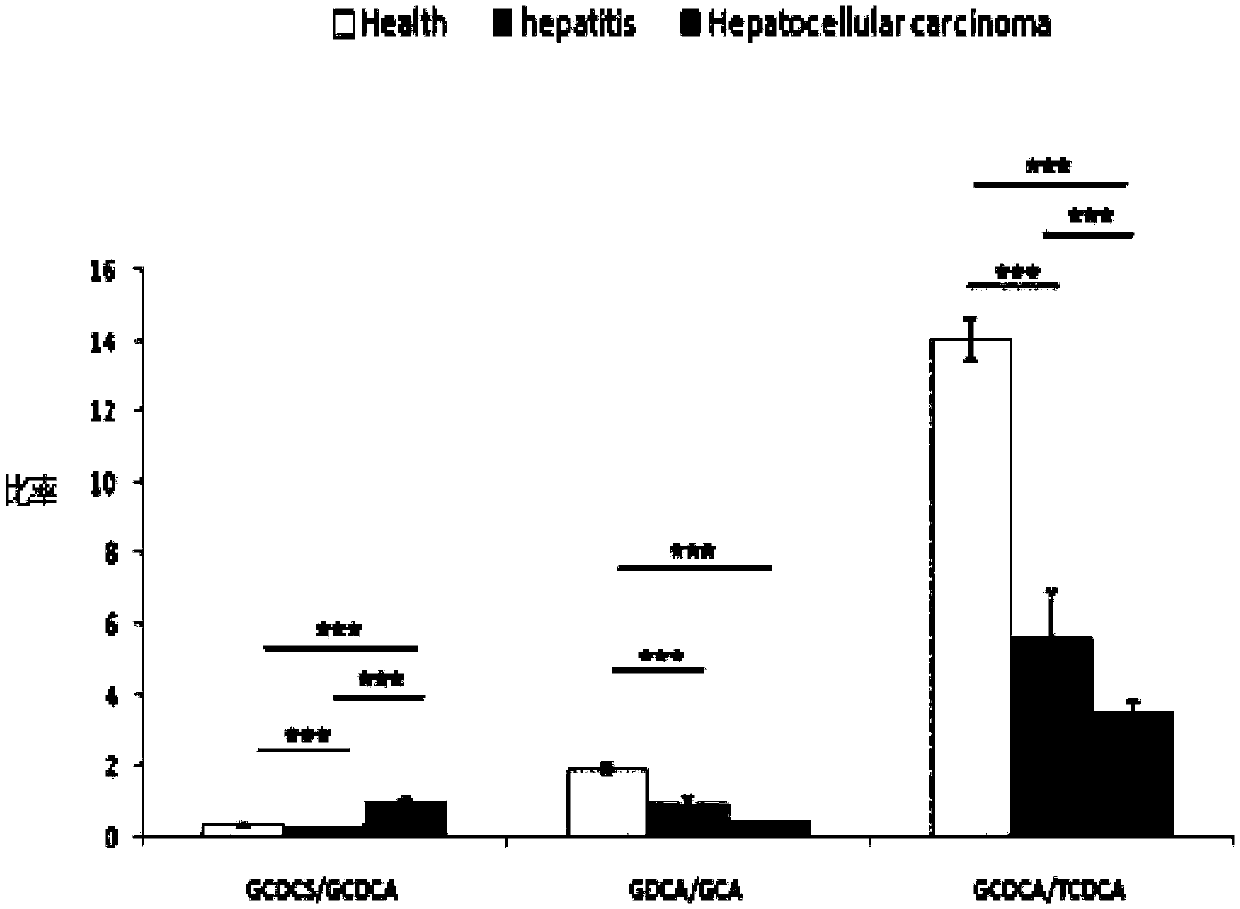
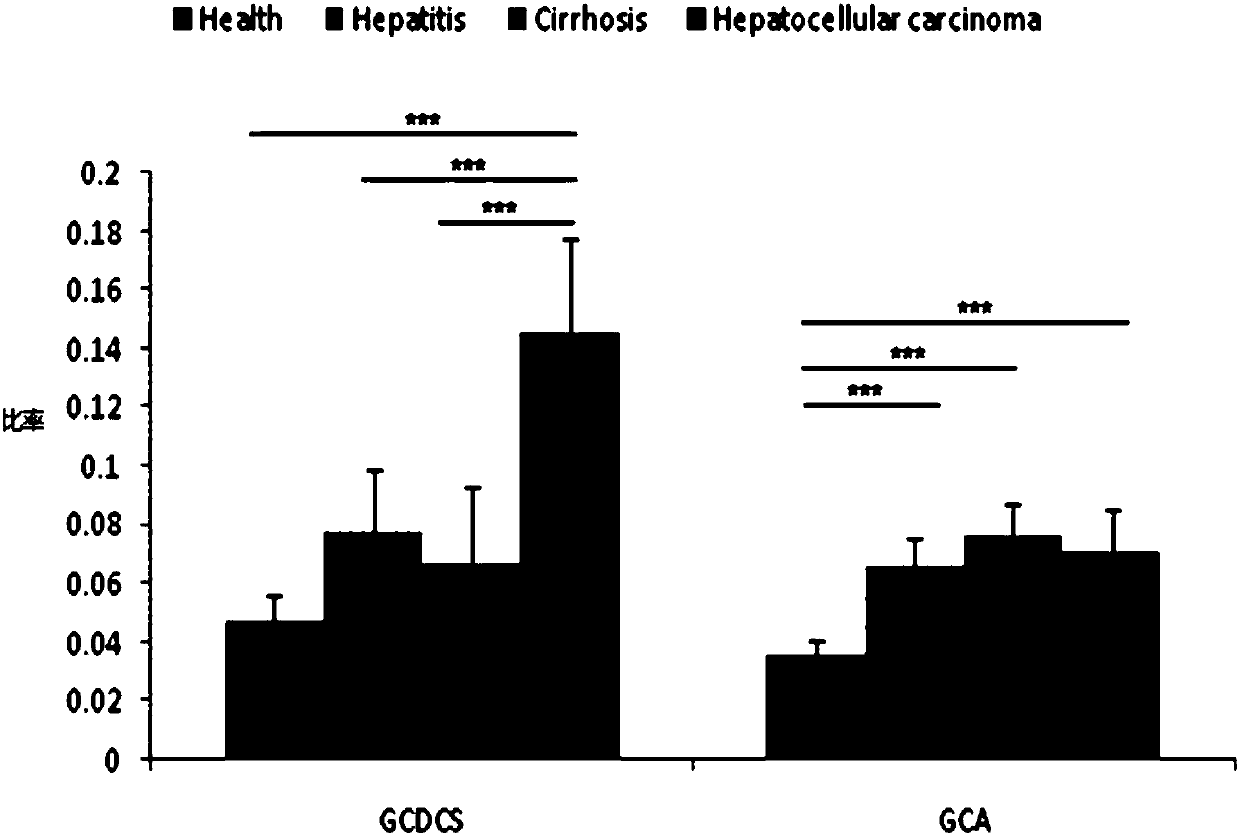
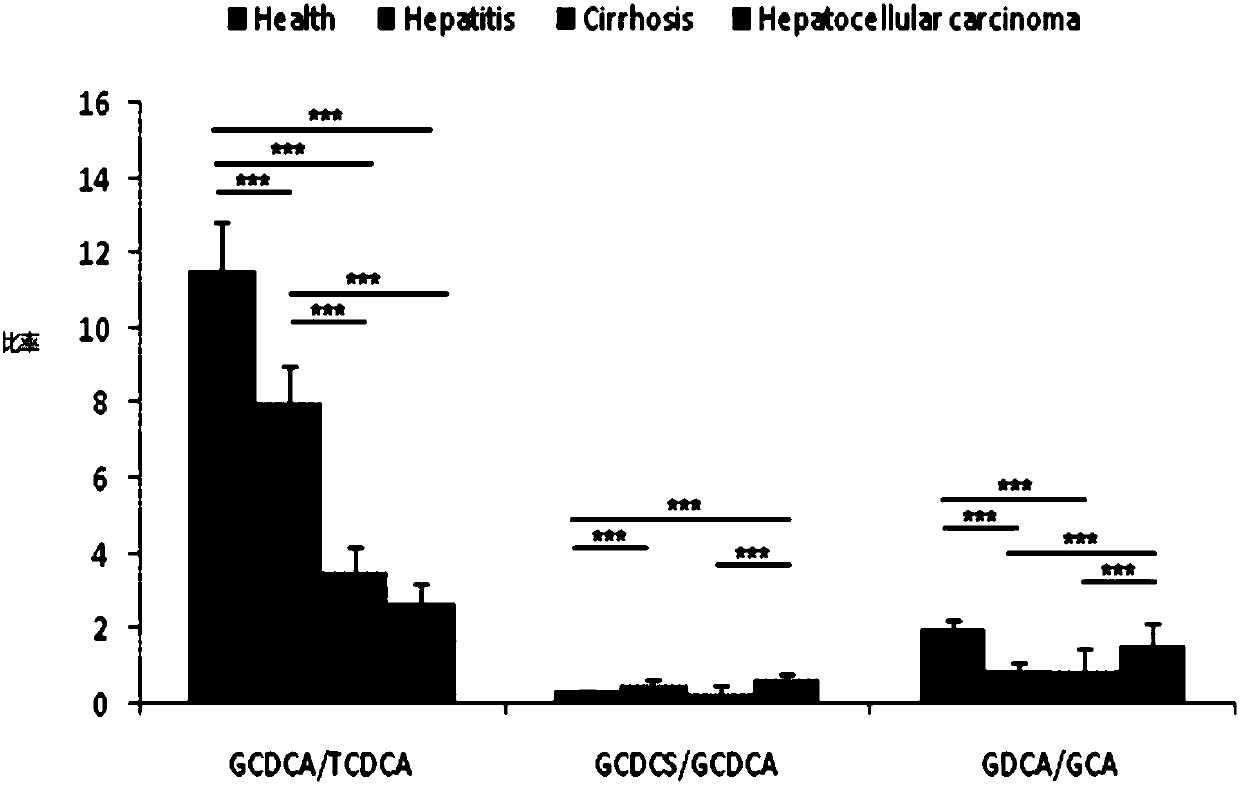
The invention provides a use of a combined serum metabolic marker in the preparation of a kit for diagnosing the progress of hepatopathy, the kit, and a method using the kit to screen serum metabolicmarkers, and relates to the technical field of serum metabolic markers. The combined serum metabolic marker is mainly composed of sodium glycochenodeoxycholate sulfate, glycochenodeoxycholic acid, glycodesoxycholic acid, glycocholic acid and taurochenodeoxycholic acid. The kit executes combined screening detection on the content of above serum metabolites in serum in order to realize assisted diagnosis of the progress of the hepatopathy of hepatopathy patients, so the difficult monitoring problem of the occurrence and development of the hepatopathy in existing clinic therapy of the hepatopathyis effectively alleviated.
Owner:HANGZHOU HEALTH BANK MEDICAL LAB CO LTD
Levofloxacin lactate liposome sodium chloride injection and preparation method thereof
InactiveCN101642434ASolve the problem of yellowing colorGuarantee product qualityAntibacterial agentsOrganic active ingredientsAntioxidantCholesterol
The invention provides a levofloxacin lactate liposome sodium chloride injection and a preparation method thereof. The levofloxacin lactate liposome sodium chloride injection is characterized by beingprepared with the following components according to parts by weight: 1 part of levofloxacin lactate, 5-15 parts of phospholipid, 0.5-1.5 parts of cholesterol, 0.5-2 parts of deoxycholate, 2-10 partsof sodium chloride and 0.5-1.5 parts of antioxidant. The invention adopts specific auxiliary materials to proportion with the original auxiliary materials and adopts the reverse phase evaporation method to prepare the levofloxacin lactate liposome sodium chloride injection of the invention, solving problems of unqualified levofloxacin lactate injection clarity and insoluble particles, and yellowing of solution color in the prior art. The invention provides a stable levofloxacin lactate liposome sodium chloride injection featuring long-time laying aside.
Owner:HAINAN YONGTIAN PHARMA INST
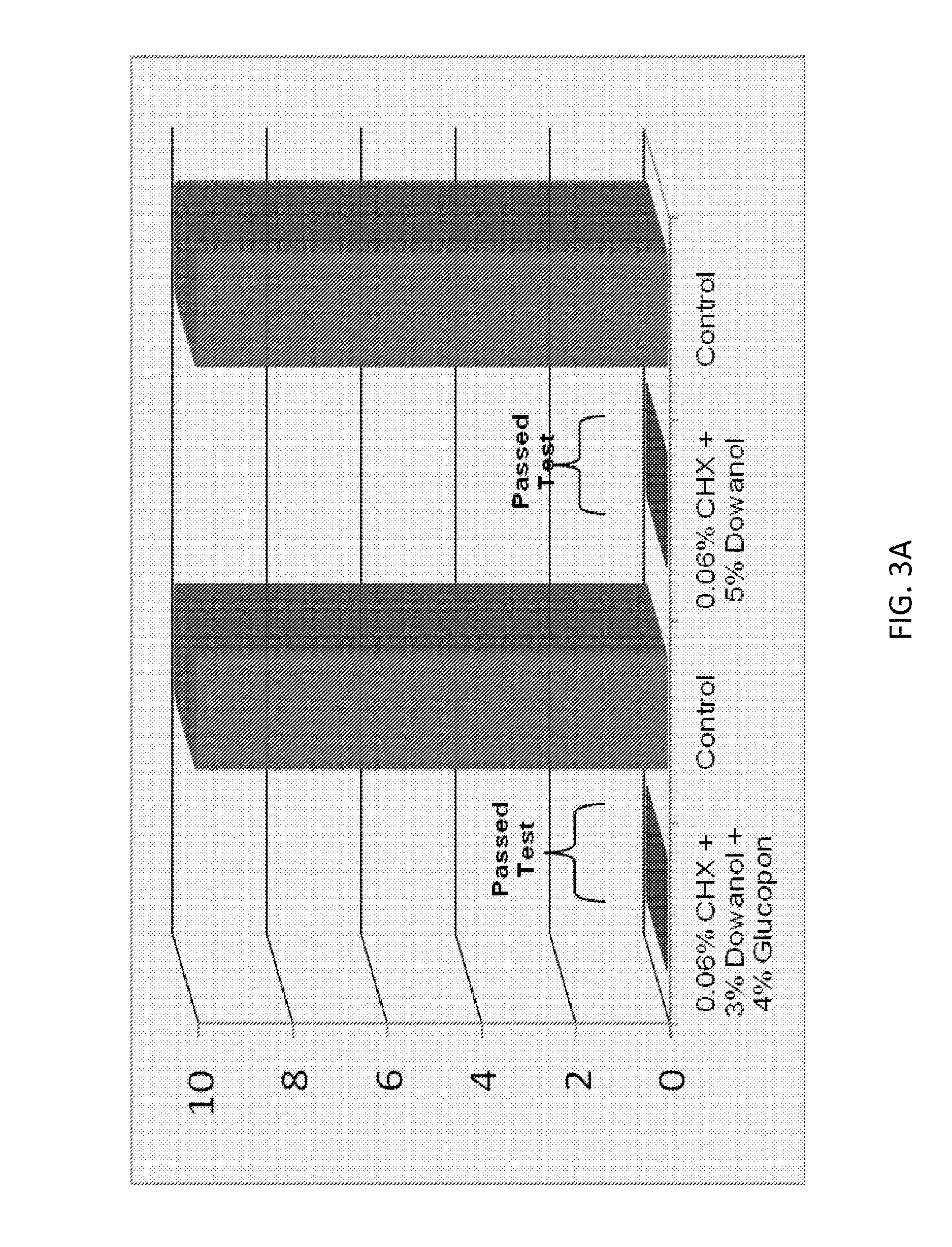
Antimicrobial solutions
ActiveUS9565857B2High activityEnhances the activity of the combination CHXOrganic active ingredientsBiocideGlycerolPharmacology
The present invention provides antimicrobial solutions that in certain cases comprise a biguanide and a glycol ether and, in some cases, optionally also includes combinations of at least one an alcohol, at least one chelator, glycerol, deoxycholate, and / or at least one alkylpolyglucoside. In certain aspects the invention comprises a biguanide and deoxycholate or a combination of chelator, ethanol, and alkylpolyglucoside. Also provided are methods for rapidly killing and / or reducing bacteria, fungi, or virus from surfaces, for example, including surfaces of indwelling medical devices and organic surfaces such as skin and sutures, and inorganic surfaces such as medical equipment, pipelines etc.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
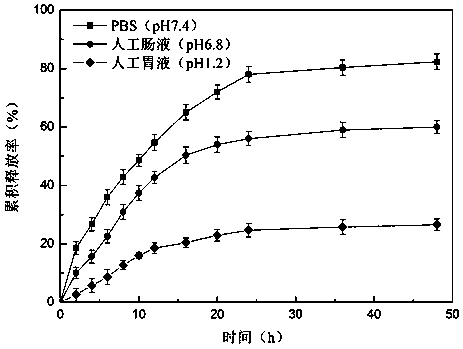
Preparation method of oleanolic acid slow-releasednano-microcapsule
ActiveCN110327311ASimple designGood choiceAntibacterial agentsOrganic active ingredientsSolubilityGastric juices
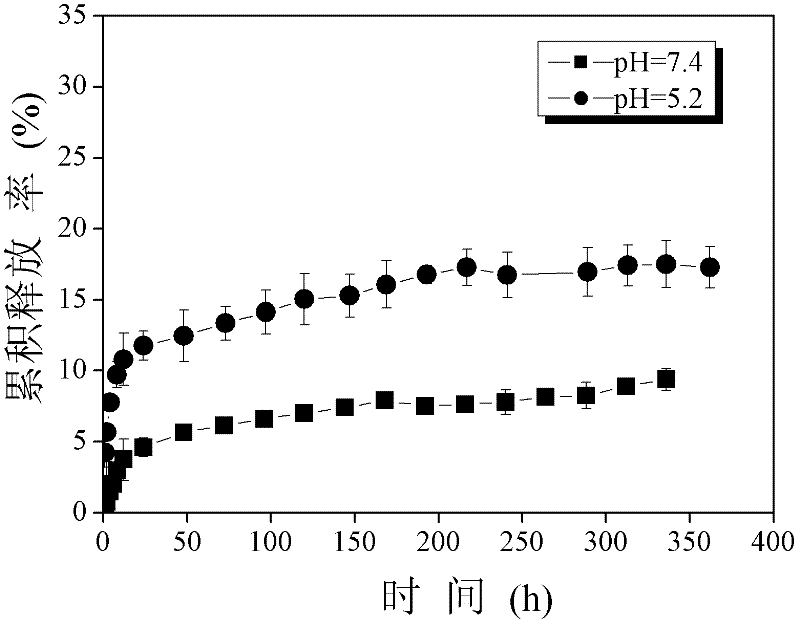
The invention provides a preparation method of an oleanolic acid slow-releasednano-microcapsule. The aim is to design and optimize oleanolic acid (OA) nanoparticles with poor water solubility to improve the oral bioavailability of the oleanolic acid (OA) nanoparticles and prolong the duration of the treatment drug level. Nanoparticle wall material is an amphiphilic polymer formed by hydrophilic chitosan oligosaccharide and hydrophobic deoxycholic acid. The particle size of nanoparticles with different wall materials is 200-400nm, and distribution is uniform. Releasing in vitro experiment is conducted in simulated gastrointestinal tract environment, results show that the oleanolic acid nanocapsule is released slowly in the simulated gastric juice and gradually released in the intestinal juice; explosive releasing is showed in early stage of releasing in the PBS solution, and then releasing is slowly; and the oleanolic acid nanocapsule is released significantly in each solution. The oleanolic acid controlled-release nanocapsule improves the bioavailability of oleanolic acid, has obvious in vitro controlled-release effect, and can be used as an effective oral preparation for future liver injury treatment.
Owner:HARBIN INST OF TECH
Qingkailing prepn for great transfusion and its prepn process
InactiveCN101019949AOvercoming low clarityOvercome stabilityOrganic active ingredientsAntipyreticHyodeoxycholic acidHydrolysis
The Qingkailing preparation for great transfusion is prepared with cholic acid 1.2-2.0 weight portions, hyodeoxycholic acid 1.4-2.4 weight portions, buffalo horn 9-16 weight portions, baicalin 1.8-3.2 weight portions, nacre 18-32 weight portions, cape jasmine 9-16 weight portions, isatis root 75-125 weight portions, and honeysuckle 23-38 weight portions. It is prepared through extraction, hydrolysis, mixing, filtering, active carbon treatment, sterilizing and other steps. The Qingkailing preparation for great transfusion has high clarity, high stability, high safety and other advantages, and is suitable for medical care personnel to use.
Owner:TSINGHUA UNIV
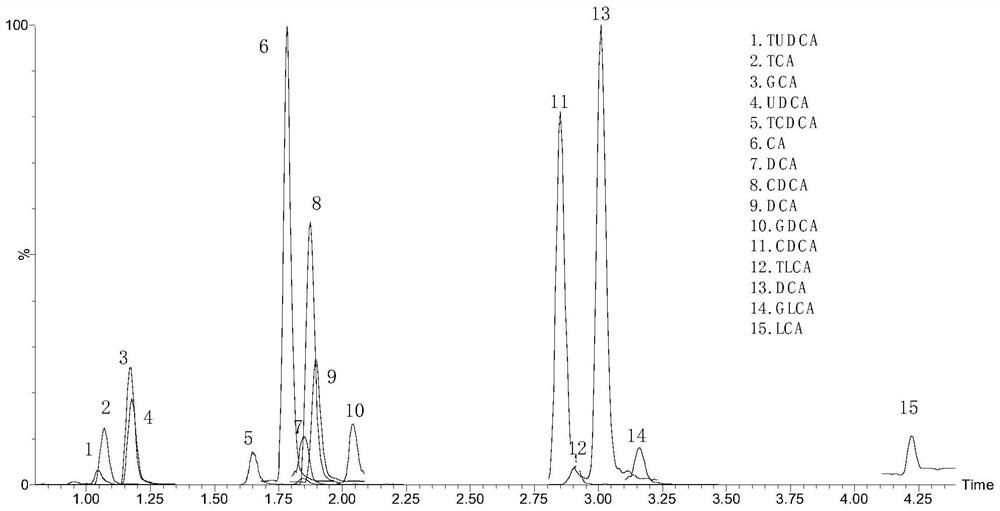
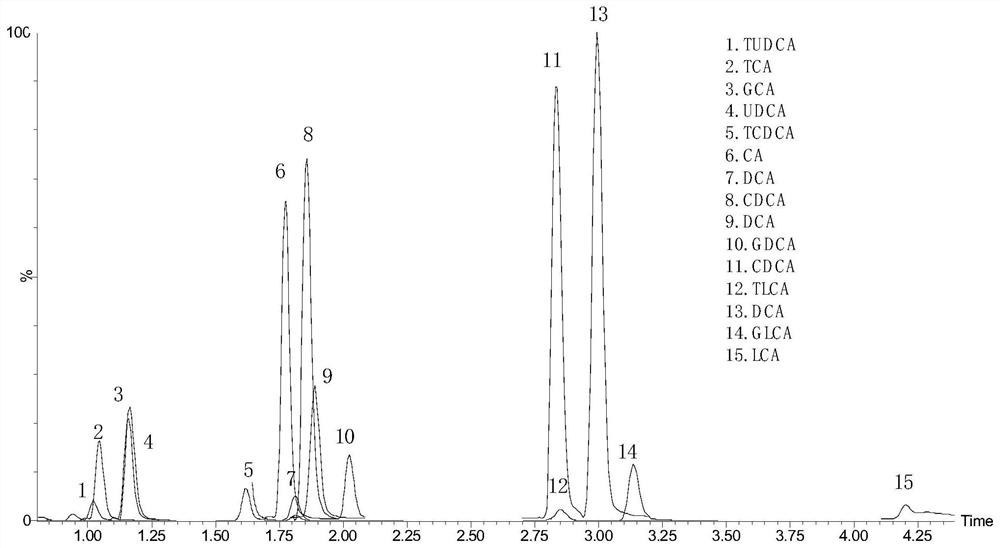
Kit for detecting 15 bile acids in serum and application of kit
The invention discloses a kit for detecting 15 bile acids in serum and an application of the kit, and belongs to the technical field of blood detection. The 15 bile acids comprise a cholic acid, a glycocholic acid, a taurocholic acid, a lithocholic acid, a glycolithocholic acid, a taurolithocholic acid, a deoxycholic acid, a glycodeoxycholic acid, a taurodeoxycholic acid, a chenodeoxycholic acid,a glycochenodeoxycholic acid, a taurochenodeoxycholic acid, an ursodeoxycholic acid, a glycoursodeoxycholic acid and a tauroursodeoxycholic acid. The kit comprises an eluent, a calibration product solution, a mixed internal standard working solution, a protein precipitant and a quality control product. When the kit is used for detecting the bile acids in the serum, a to-be-detected sample does notneed derivatization treatment, pretreatment is simple, a sample dosage is small, sensitivity is high, specificity is strong, more types can be detected, the 15 types of bile acid can be simultaneously detected within 6.5 minutes, and the kit can be used for clinical diagnosis and health assessment of serum bile acids.
Owner:NANJING PINSHENG MEDICAL TECH CO LTD
Preparation method and application of autonomous nano generator pharmaceutical composition based on probiotic spores
InactiveCN110812493AAchieve synergyEfficient killingMaterial nanotechnologyBacteriaBiotechnologyTumor therapy
The invention relates to a preparation method and application of an autonomous nano generator pharmaceutical composition based on probiotic spores, can effectively solve the problems that a drug is easy to degrade in the stomach after being orally taken and the dilemma that the drug is easy to take and difficult to transport in oral administration at present, and provides construction of an oral administration system. The method comprises the following steps: firstly culturing Bacillus coagulans to generate spores, then modifying deoxycholic acid which can be combined with a bile acid receptoron the spore surfaces through amidation reaction, and then physically loading anti-tumor drugs adriamycin and sorafenib to obtain the autonomous nano generator pharmaceutical composition based on theprobiotic spores. The composition has the advantages of simple preparation method, low production cost, stable gastric acid environment and the like, retains the regeneration capacity of the drug-loaded spores, integrates a biological carrier and chemotherapy, further efficiently delivers the drugs to systemic circulation, and provides technical support for tumor treatment of oral administration.
Owner:ZHENGZHOU UNIV
A tacrolimus capsule
ActiveCN106880617AEvenly dispersedPromote dissolutionOrganic active ingredientsPharmaceutical non-active ingredientsDigestionDesoxycholic acid
A tacrolimus capsule is disclosed. Tacrolimus, hydroxypropyl-beta-cyclodextrin and deoxycholic acid are dissolved into absolute ethanol, then the obtained solution is granulated on pharmaceutically acceptable auxiliary materials and dried, and a capsule is filled. Compared with the prior art, the preparing process is simple, medicine dispersion is uniform, a production procedure is smooth and medicine digestion is rapid.
Owner:SHANDONG NEWTIME PHARMA
Preparation method and application of deoxycholic-acid-modified silver nanoparticle solution
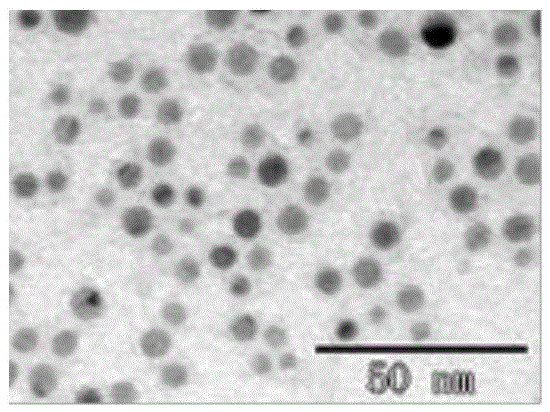
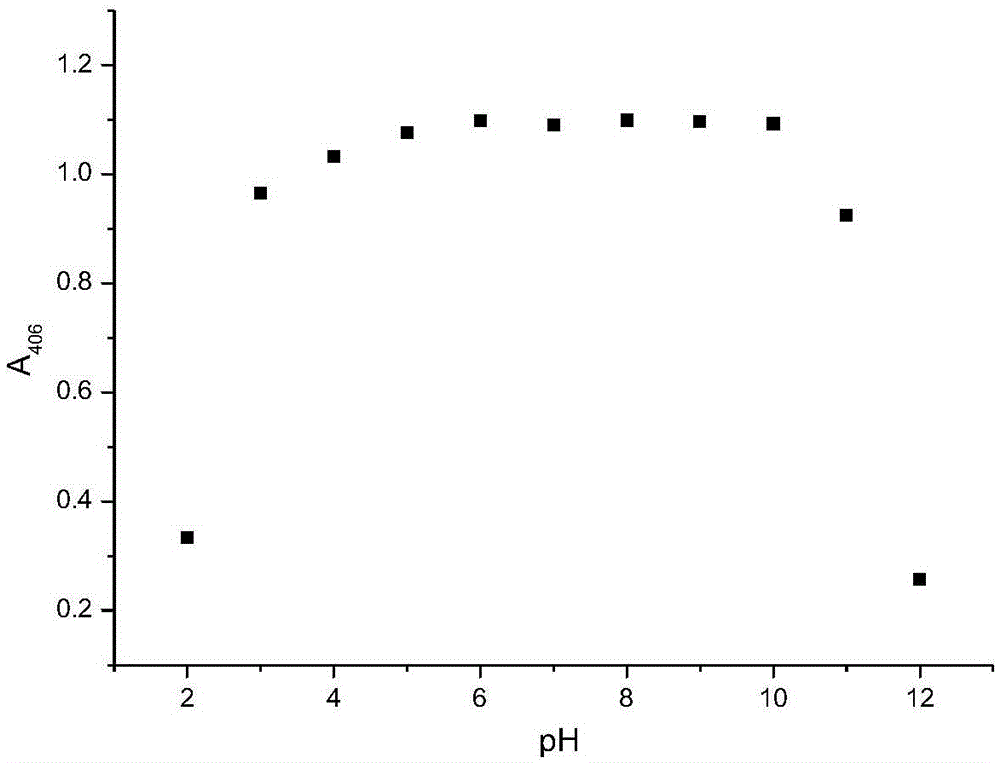
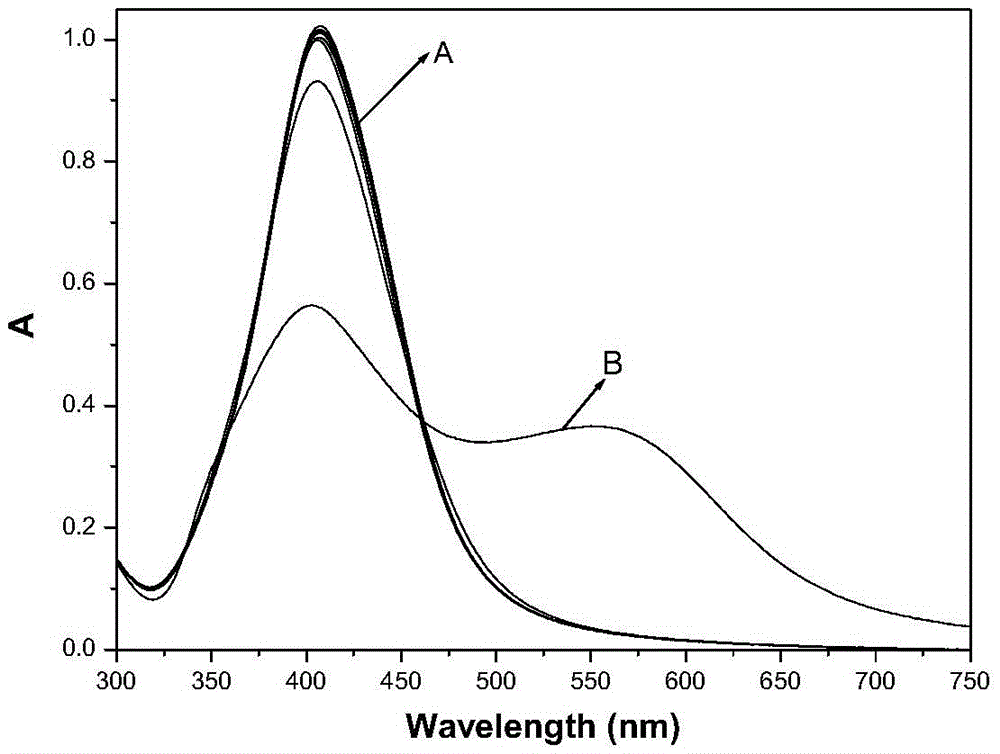
ActiveCN105548157AThe preparation method is simple and feasibleProcess conditions are stableMaterial analysis by observing effect on chemical indicatorAqueous solutionDesoxycholic acid
The invention discloses a preparation method and application of a deoxycholic-acid-modified silver nanoparticle solution. The preparation method of the deoxycholic-acid-modified silver nanoparticle solution comprises the following steps: step 1, preparing a deoxycholic acid solution; step 2, adding AgNO3 into 90 parts by weight of water to form an AgNO3 aqueous solution with a concentration of 5*10<-5>-5*10<-3> mol / L; and step 3, adding 0.005-0.012 part by weight of NaBH4 into the AgNO3 aqueous solution while performing stirring, performing continuous stirring for 1-5 min, adding 4.5-9 parts by weight of the deoxycholic acid solution, and performing continuous stirring for 0.5-5 h to obtain the deoxycholic-acid-modified silver nanoparticle solution. The prepared deoxycholic-acid-modified silver nanoparticle solution has good monodispersion, uniform particles, stable properties, and excellent optical performance.
Owner:杭州天时亿科技有限公司
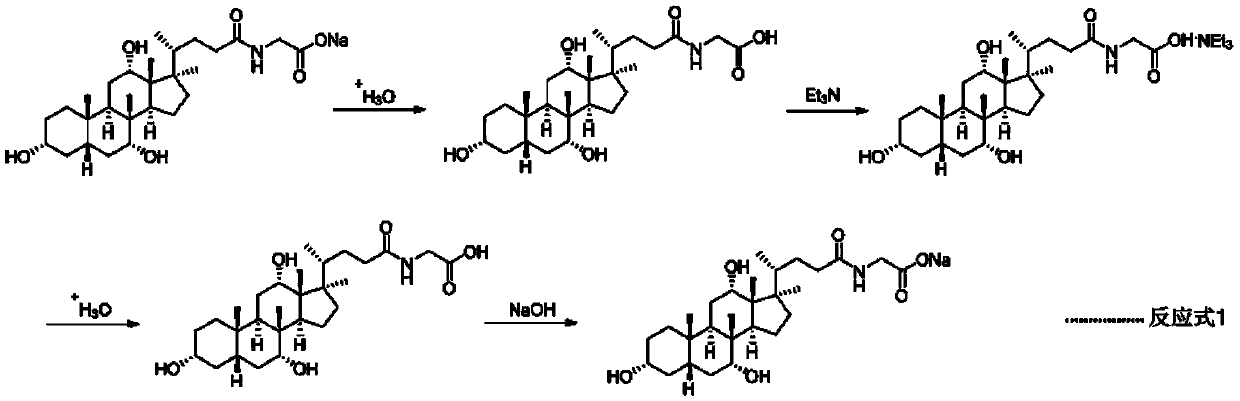
Refining method of glycochenodeoxycholic acid sodium
The invention relates to the technical field of organic synthesis, in particular to a refining method of glycochenodeoxycholic acid sodium. The method includes the steps of S1, adding commercially-available glycochenodeoxycholic acid sodium to water, adjusting the pH value into the weak acid range with an acid water solution, conducting extracting through an organic solvent, adjusting the pH valueof the water phase into the strong acid range with the acid water solution, and conducting filtering to obtain glycochenodeoxycholic acid; S2, heating and stirring the glycochenodeoxycholic acid andorganic alkali in a solvent to form salt, and conducting cooling and filtering to obtain glycochenodeoxycholic acid organic alkali salt; S3, adjusting the pH value of the glycochenodeoxycholic acid organic alkali salt in the solvent into the strong acid range with an acid water solution, and conducting filtering, washing and drying to obtain a glycochenodeoxycholic acid refined product. The methodhas the advantages of being simple and easy to operate, stable in process, easy to control, beneficial for post-treatment, safe, friendly to the environment and the like, and can be continuously produced on a large scale.
Owner:YAOPU SHANGHAI PHARMA TECH CO LTD
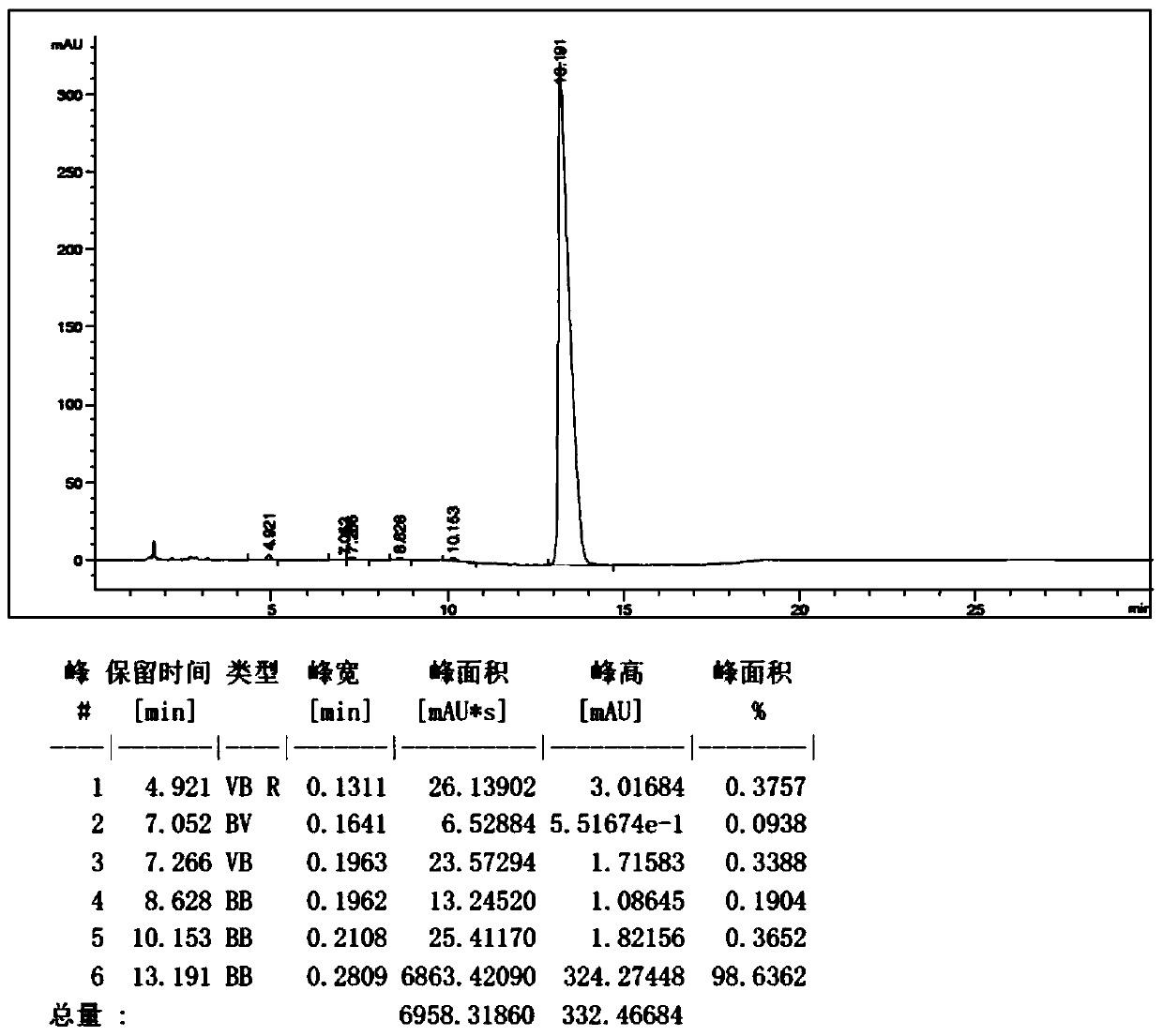
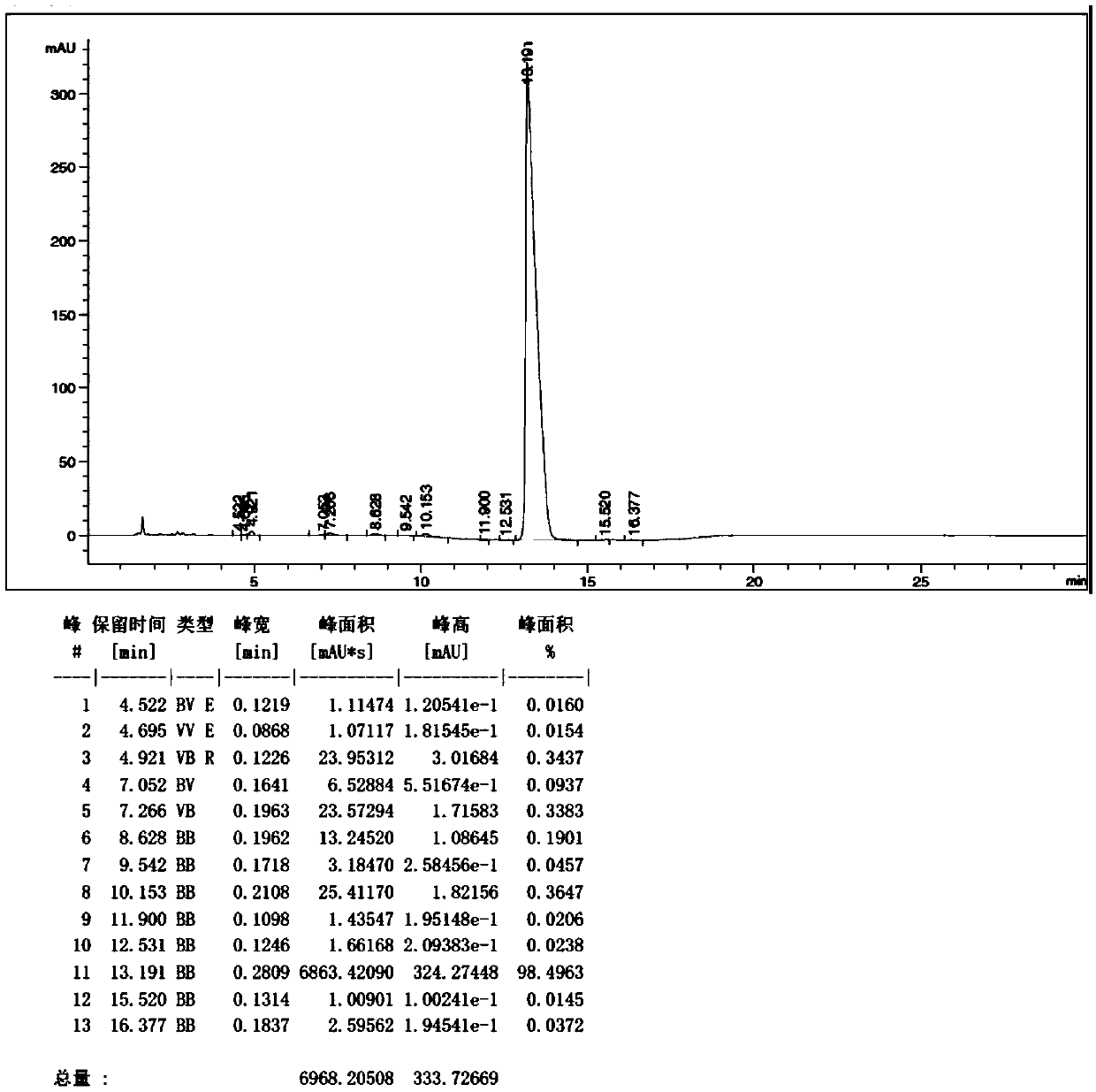
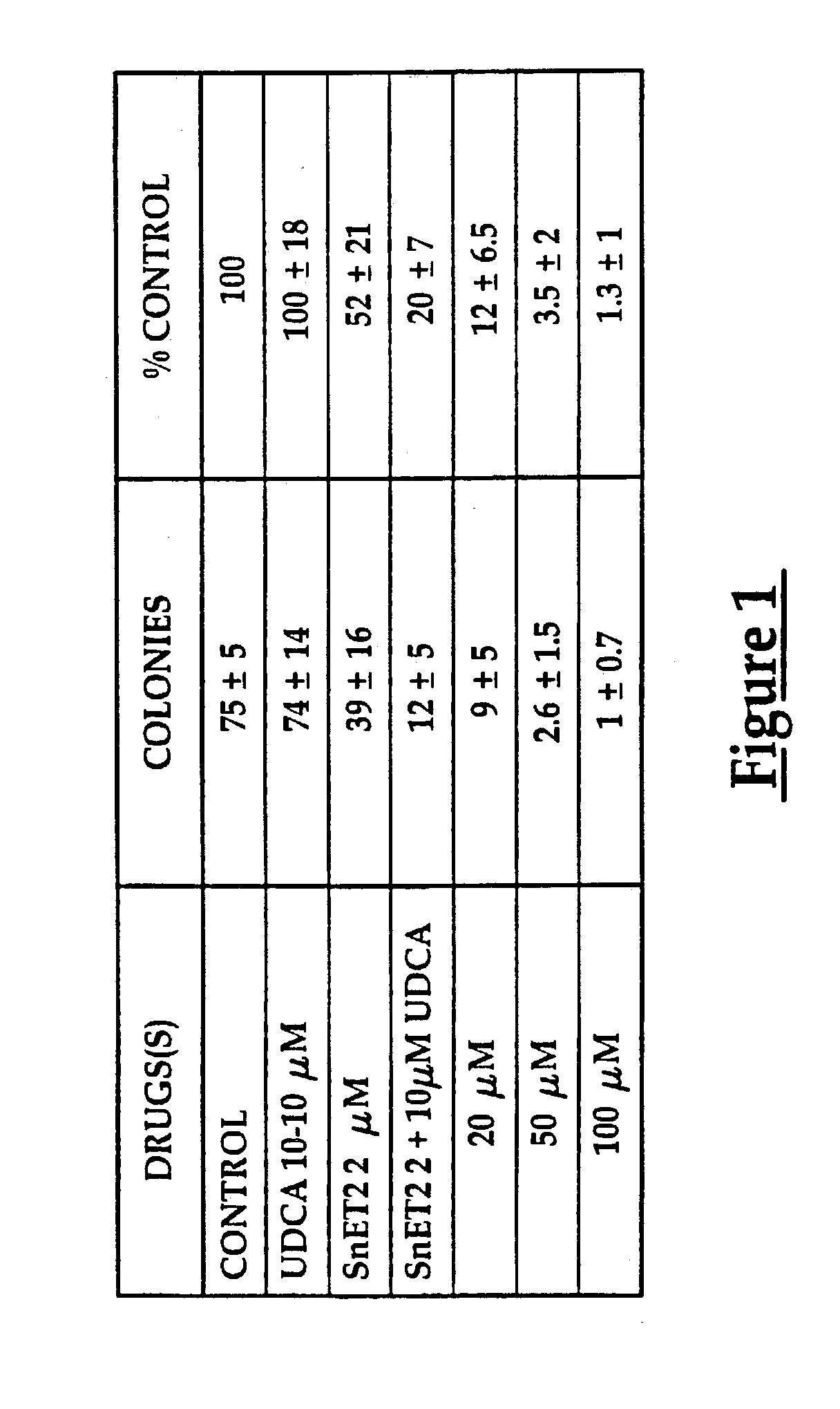
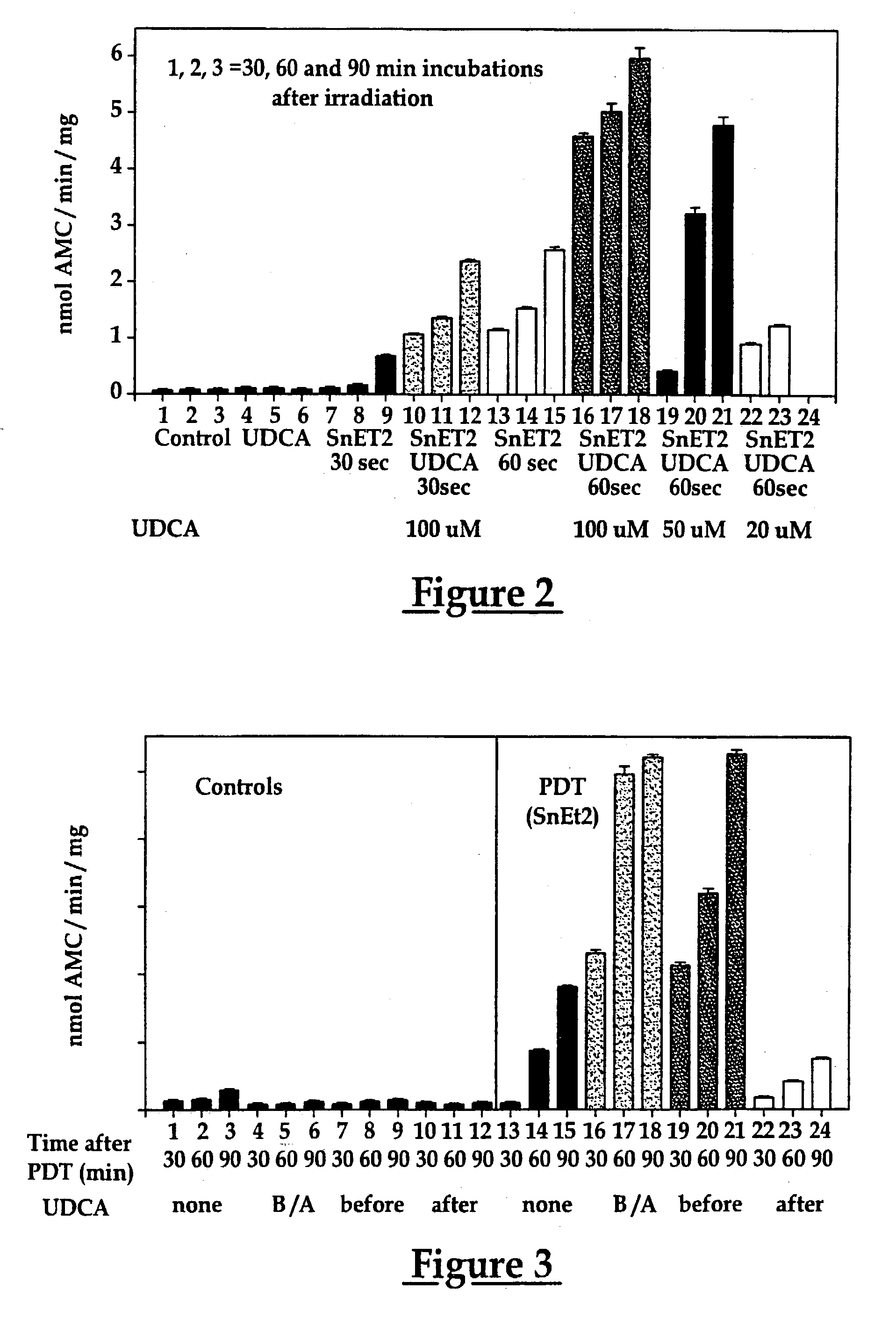
Use of ursdeoxycholic acid for potentiation of the phototoxic effect of photodynamic therapy
A method of potentiating the phototoxicity of photodynamic therapy by co-administering a photosensitizing agent with a photodynamic potentiator from the group consisting of uridioxicolic acid analogs and conjugates thereof having the photo-toxicity potentiating effect and allowing for retention of the co-administered agent and acid in the target tissue. The target tissue is then irradiated. A tool for potentiating apoptosis consists of a photosensitizing agent and a non-toxic photodynamic potentiator. Further, a method of potentiating drug induced apoptosis is provided by administering a photosensitizing agent and then decreasing the threshold of responsiveness of a target tissue to a photokilling effect of the photosensitizing agent.
Owner:KESSEL DAVID +1
Method for preparing deoxycholic acid from phytosterol
ActiveCN112877393AReduce usageEasy to operateMutant preparationMicroorganism based processesBiotechnologyChemical synthesis
The invention provides application of a mutagenic strain of which the preservation number is CCTCC (China Center For Type Culture Collection) NO: M 2020987 in preparation of deoxycholic acid. The invention provides a novel method for preparing deoxycholic acid by taking phytosterol as a raw material and combining biological fermentation and chemical synthesis, animal source extraction is not used in the method, industrial wastewater and waste residues can be used as raw materials, and the method is green and environment-friendly; the carbonyl side chain of the cholic acid compound is constructed on the side chain of the phytosterol in one step by virtue of a mycobacterium sp.NRRL B-3805 mutagenic strain biological fermentation method, so that the operation is simple, the yield is high, isomer impurities are few, and the use amount of an organic solvent is small; according to the method for preparing the deoxycholic acid compound, reaction reagents are simple and easy to obtain, reaction conditions are mild, the reaction yield is high, and the method is suitable for large-scale industrial production.
Owner:湖南醇健制药科技有限公司
Synthesis and use of Anti-tumor drug lqc-y
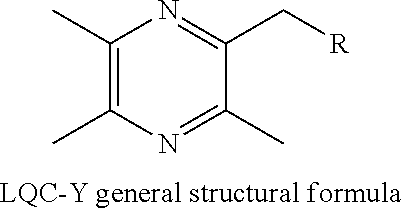
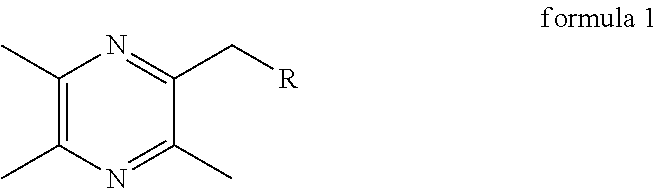
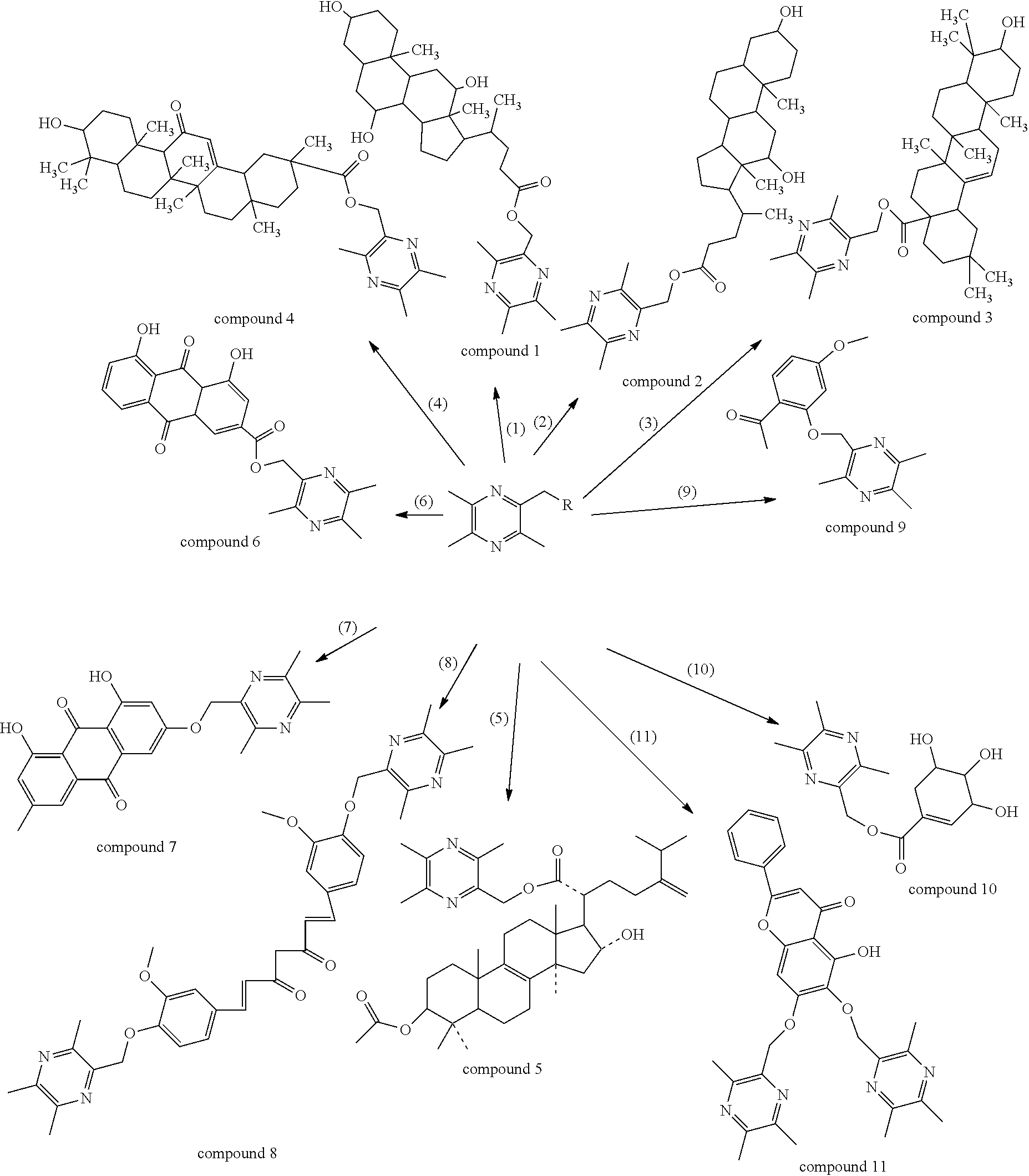
InactiveUS20140011786A1Increase spleen indexInhibit the growth of S180 tumor effectivelyOrganic active ingredientsSteroidsStructural formulaLung cancer
Disclosed are the general structural formula of LQC-Y as well as the synthesis and use thereof. Pharmacological experiments demonstrated the marked antitumor effect of such compounds. Single day administration of LQC-Y3 to mice at a maximum dose of 6000 mg / kg showed no toxicity response during the 14-day continuous observation period, indicating the high safety of the compounds, and the compounds can be used to prepare medicaments for preventing and treating carcinomas such as liver cancer, lung cancer. In the general structural formula of LQC-Y, R represents steroid compounds such as cholic acid, deoxycholic acid, ursodeoxycholic acid, chenodeoxycholic acid, and hyodeoxycholic acid and so on; triterpenoid compounds such as oleanolic acid, ursolic acid, pachymic acid, glycyrrhetinic acid and glycosides thereof and so on; emodic acid, emodin and other mono-substituted or poly-substituted structures of anthraquinone parent nucleus; baicalein, baicalin and other flavonoid; shikimic acid, mono-substituted shikimic acid or poly-substituted shikimic acid; gardenia acid and other iridoid acid derivatives; paeonol, curcumin and structural derivatives thereof.
Owner:3D MEDICINES (BEIJING) CO LTD
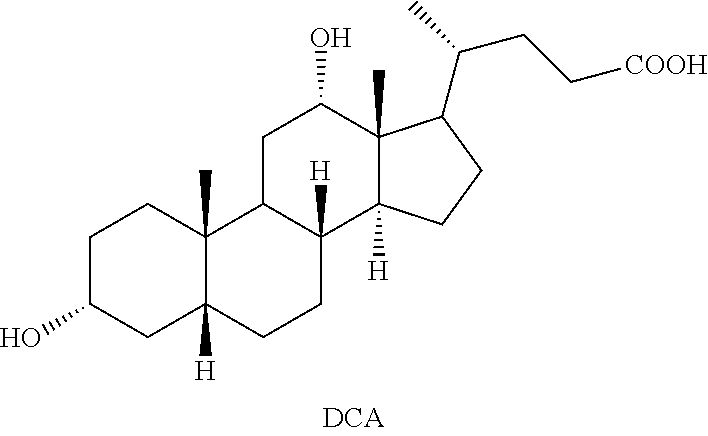
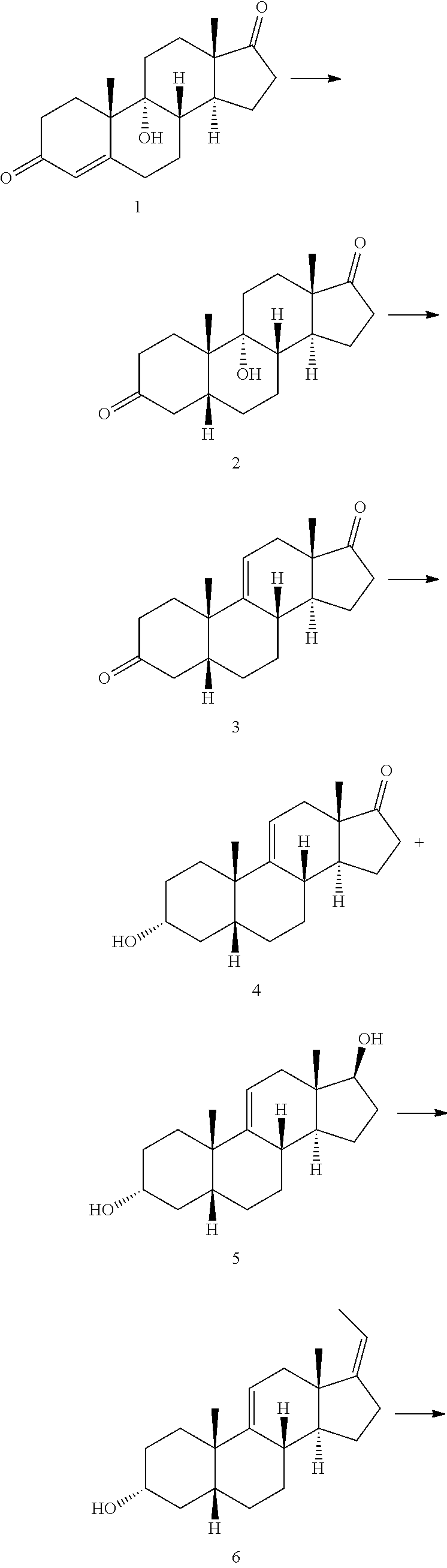
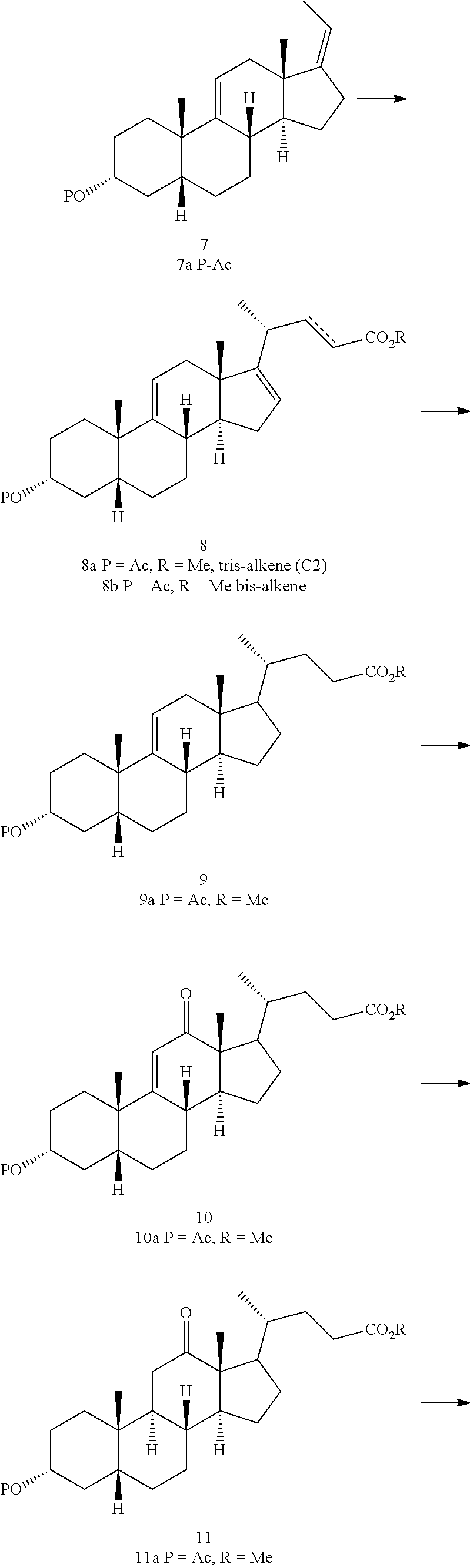
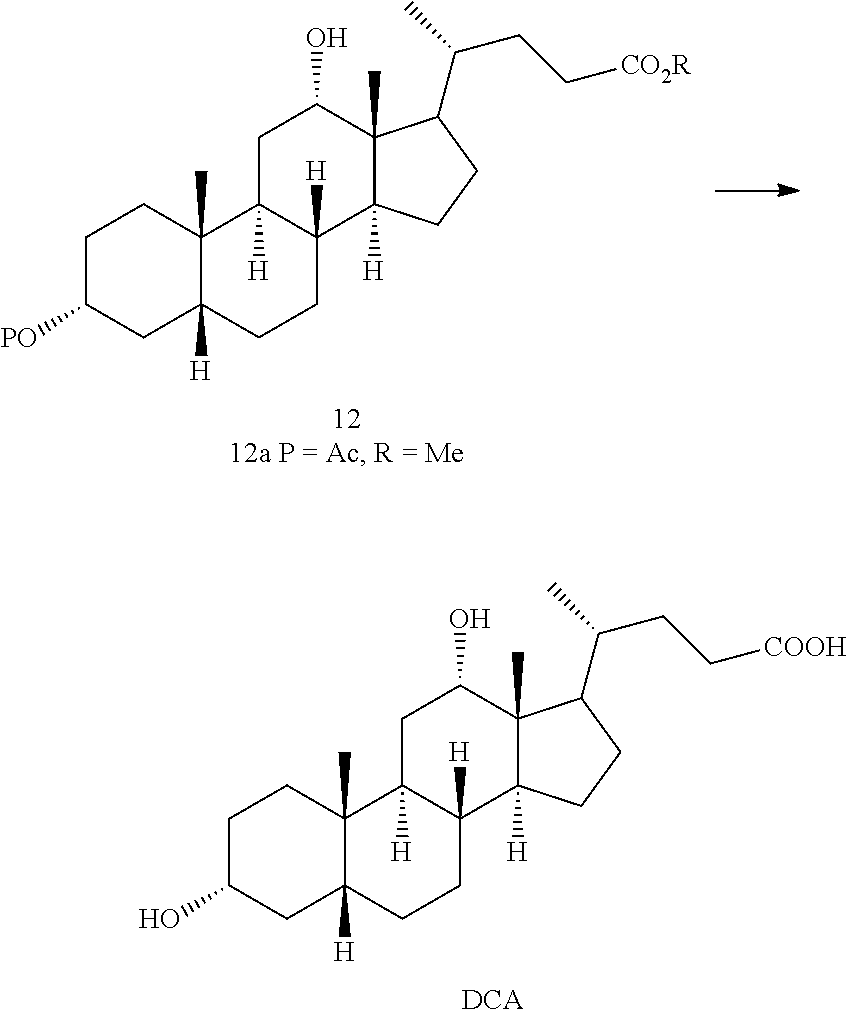
Methods for preparing deoxycholic acid
The present invention discloses method for preparing deoxycholic acid (DCA) or an ester thereof or a pharmaceutically acceptable salt thereof. Said compounds may be applied to remove a fat deposition.
Owner:NANJING NORATECH MEDICAL TECH CO LTD
Amphipathic chitosan derivative and preparation method and application thereof
InactiveCN102241790BRich sourcesPreparation reaction conditions are mildOrganic active ingredientsGenetic material ingredientsClick chemistryBackbone chain
The invention discloses an amphipathic chitosan derivative PAMAM-Cs-DCA (Poly(amido amine)-chitosan-deoxycholic acid). The PAMAM-Cs-DCA is prepared by sequentially grafting a PAMAM unit and a deoxycholic acid unit on a main chain of chitosan by click chemical reaction and amidation reaction. The preparation method has mild reaction conditions, high efficiency and selectivity. The invention also discloses an application of the amphipathic chitosan derivative in preparing an anticancer drug carrier: the amphipathic chitosan derivative forms nanomicelle which takes the PAMAM unit and chitosan asa hydrophilic shell and takes the DCA unit as a hydrophobic core by self assembly in a water solution, wherein hydrophobic anticancer drugs can be coated in the core, and the shell can be compounded with pDNA (plasmid deoxyribonucleic acid) to realize co-transmission of the drugs and genes. Due to the unique molecular structure, the amphipathic chitosan derivative has potential application valuesin the fields of gene therapy, controlled release of drugs, tissue engineering and the like.
Owner:SUN YAT SEN UNIV
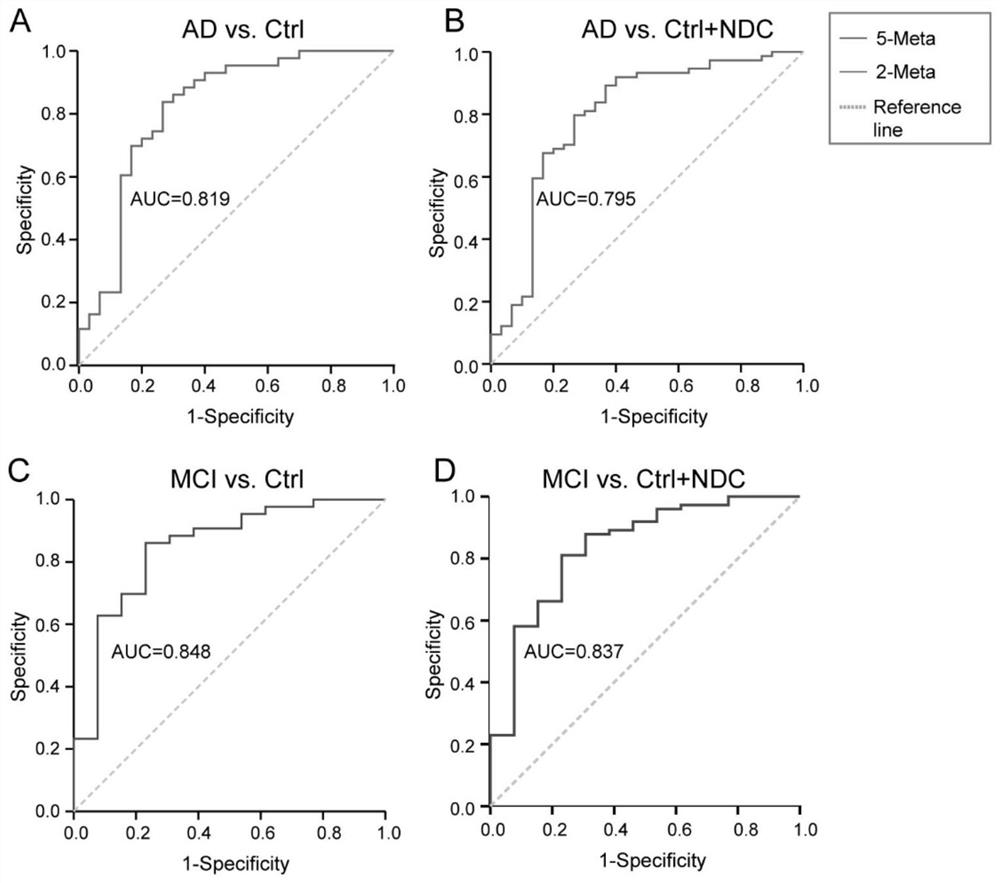
A group of biomarkers and applications thereof for diagnosing AD in a subject or determining the risk of developing AD in a subject
Owner:FIRST AFFILIATED HOSPITAL OF DALIAN MEDICAL UNIV
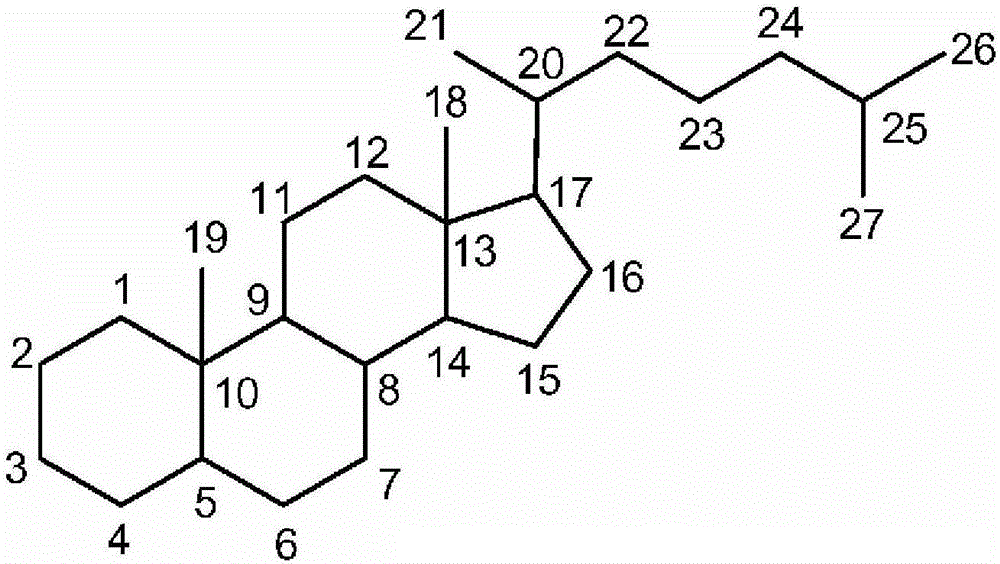
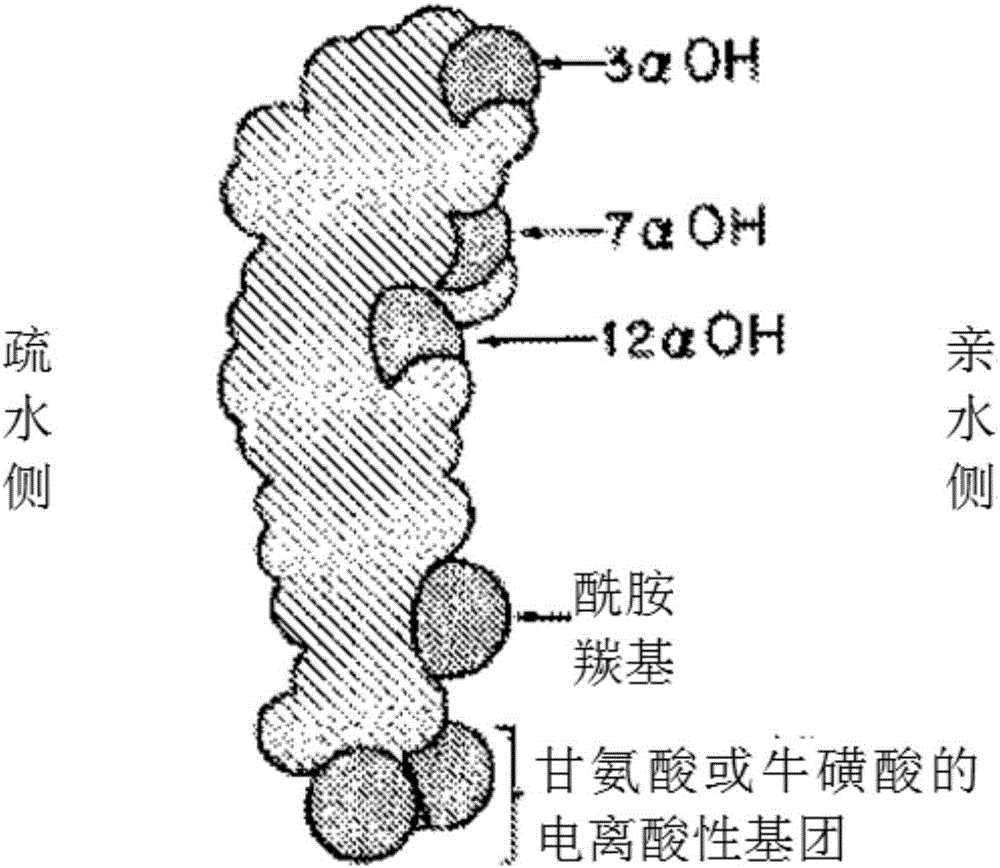
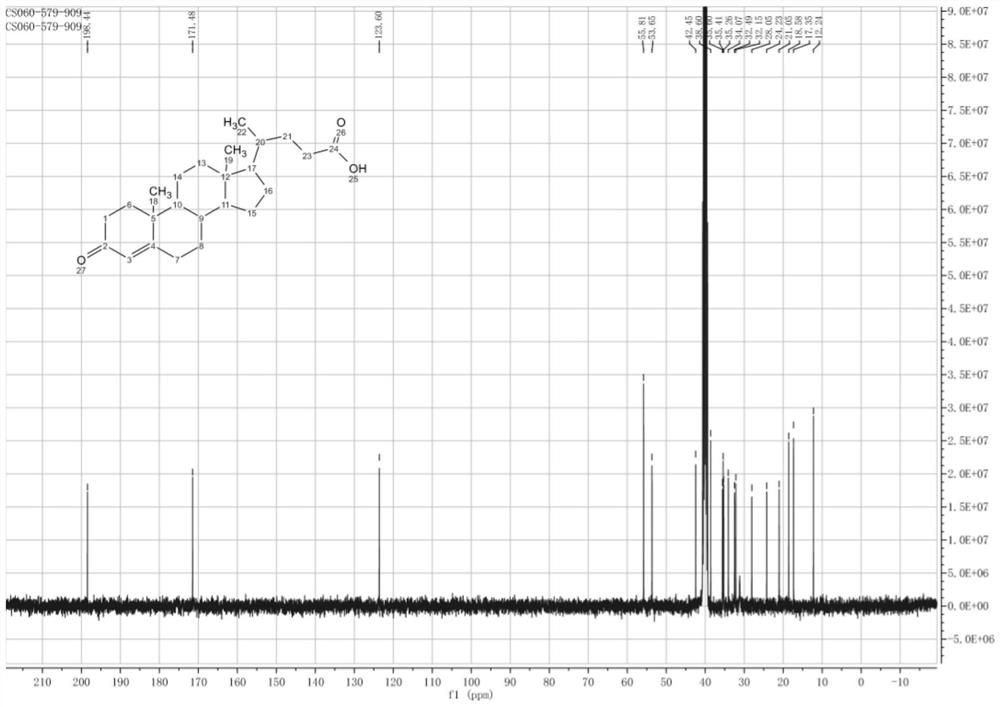
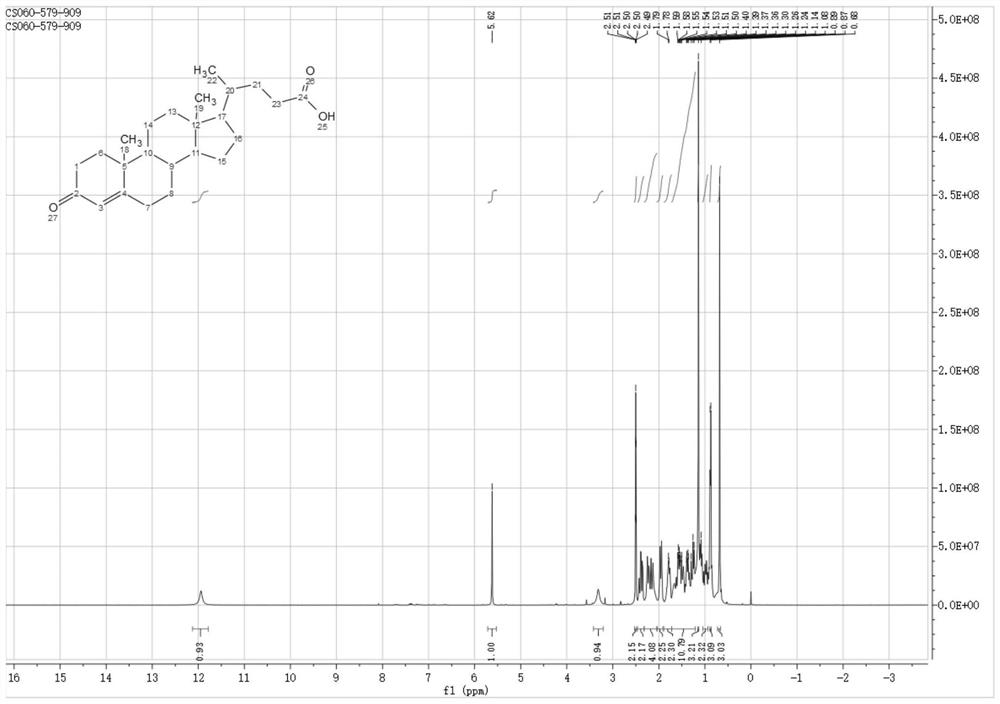
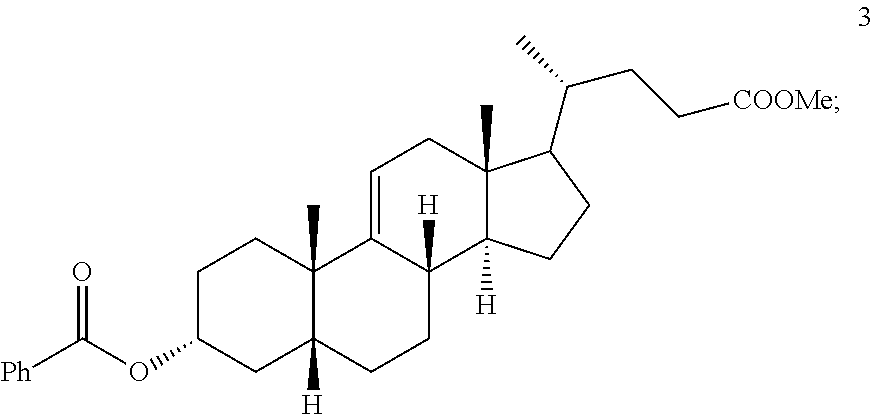
A kind of synthetic method of deoxycholic acid
The invention discloses a synthetic method of deoxycholic acid. The method is characterized by comprising the following steps: (A) preparing cholate; (B) preparing 3 alpha, 12 alpha-diacetyl base-7 alpha-hydroxyl cholate; (C) preparing 3 alpha, 12 alpha-diacetyl base-7-oxocholate; (D) preparing 7-oxocholic acid; and (E) preparing deoxycholic acid. The purpose of the invention is to provide the synthetic method of the deoxycholic acid with simple process, comparatively lower production cost, high deoxycholic acid yield and convenience in industrial production in order to overcome defects in the prior art. By using cholic acid as a raw material and selecting thionyl chloride, morpholine and ammonium chloride as catalysts, the synthetic method of the deoxycholic acid, disclosed by the invention, has the advantages of simplicity in process, strong reaction specificity, high yield and great convenience in industrial production. Through adoption of the synthetic method disclosed by the invention, the deoxycholic acid is prepared with low cost.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
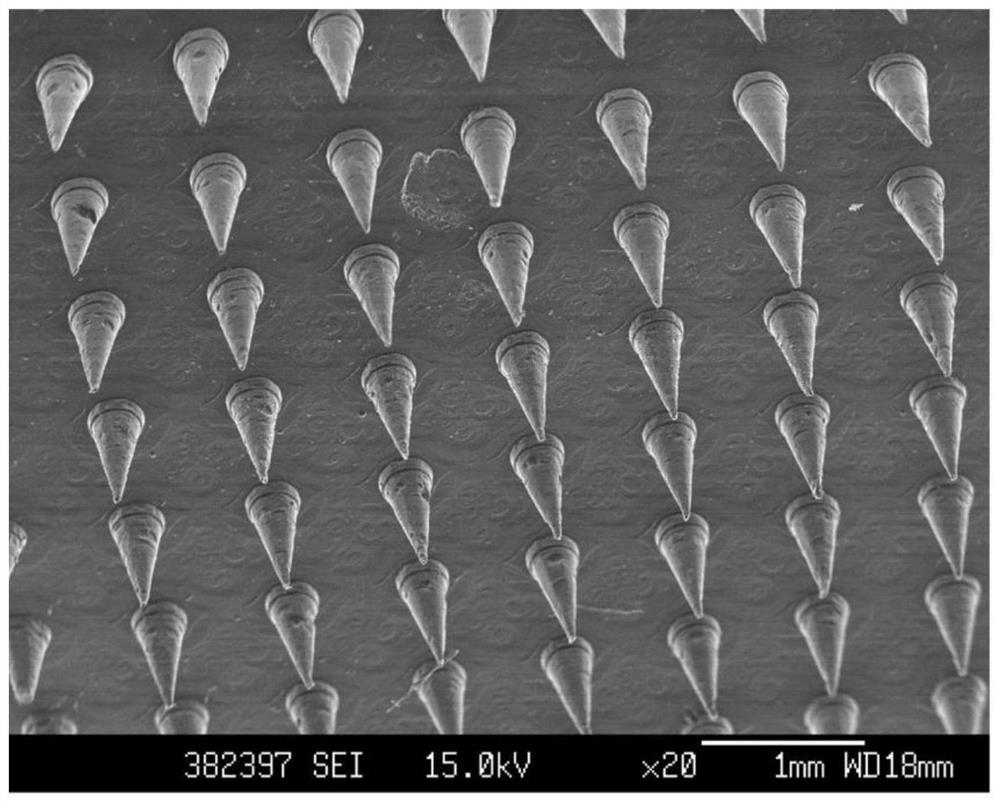
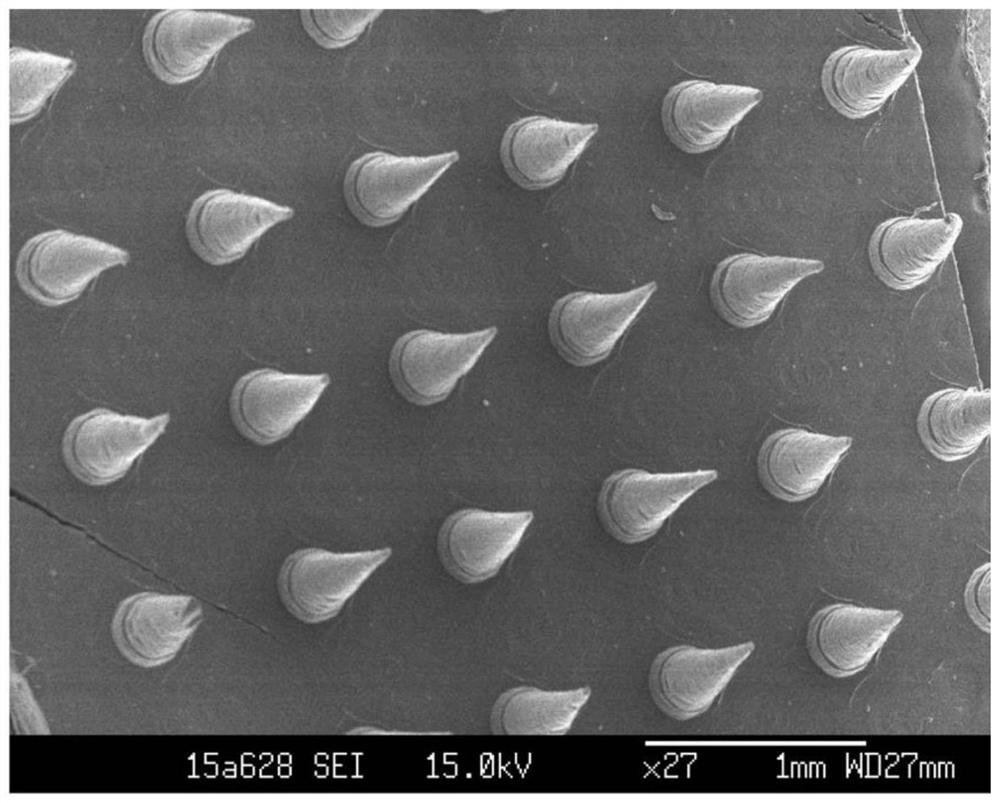
Soluble microneedle patch and preparation method thereof
PendingCN114306917AImprove bioavailabilityIncrease biological transdermalMedical devicesPolythylene glycolHydroxystearic Acid
The invention relates to a soluble microneedle patch and a preparation method thereof. The soluble microneedle patch comprises a substrate and a needle body on the substrate, the needle body comprises the following components: a high-molecular polymer framework material, polypeptide or protein drugs, a penetration enhancer and a stabilizer; the substrate comprises the high-molecular polymer framework material; the penetration enhancer is at least one of 15-hydroxystearic acid polyethylene glycol ester, Tween 80, deoxycholate, nicotinamide and amino acid. The microneedle patch is used for delivering polypeptide or protein drugs, the intradermal release and permeation speed of the polypeptide or protein drugs is obviously improved, the concentration of the polypeptide or protein drugs entering blood circulation is obviously increased, and the in-vivo bioavailability of the polypeptide or protein drugs after being delivered and administered through the microneedle is improved.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
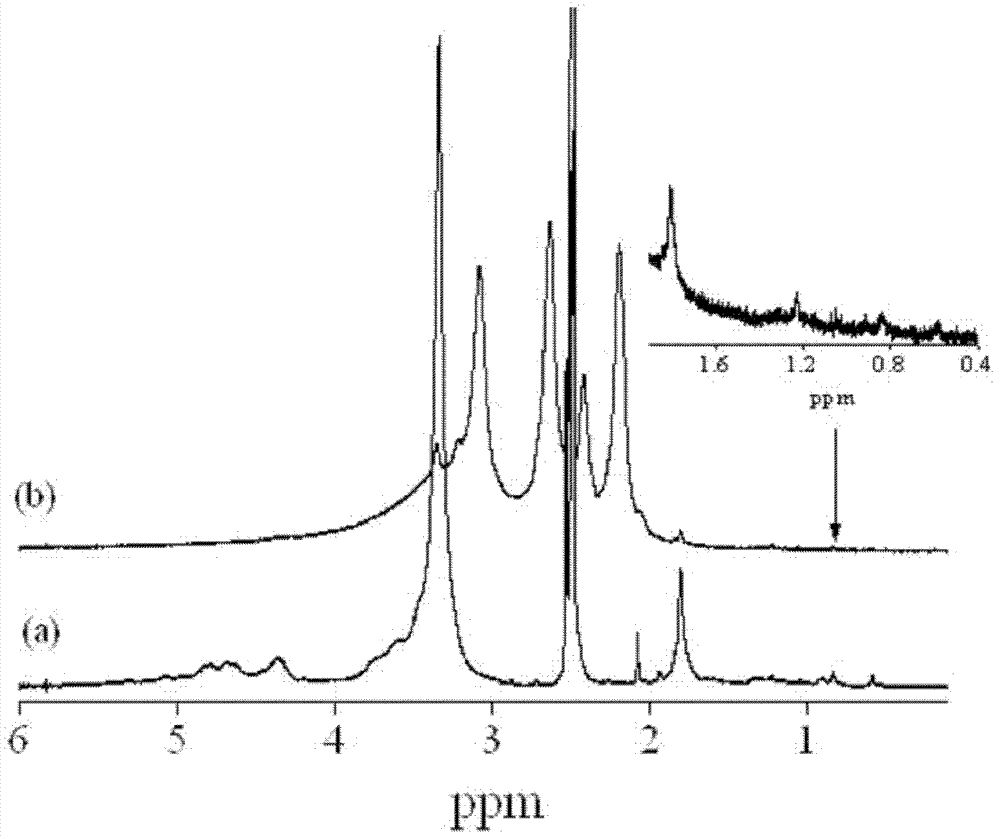
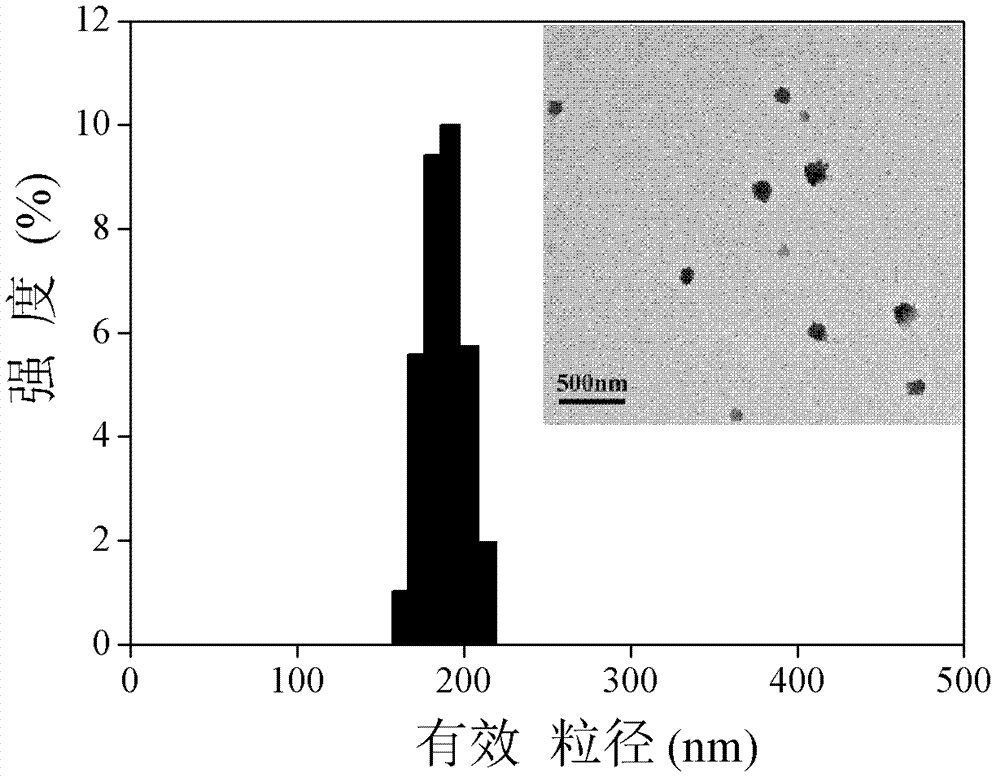
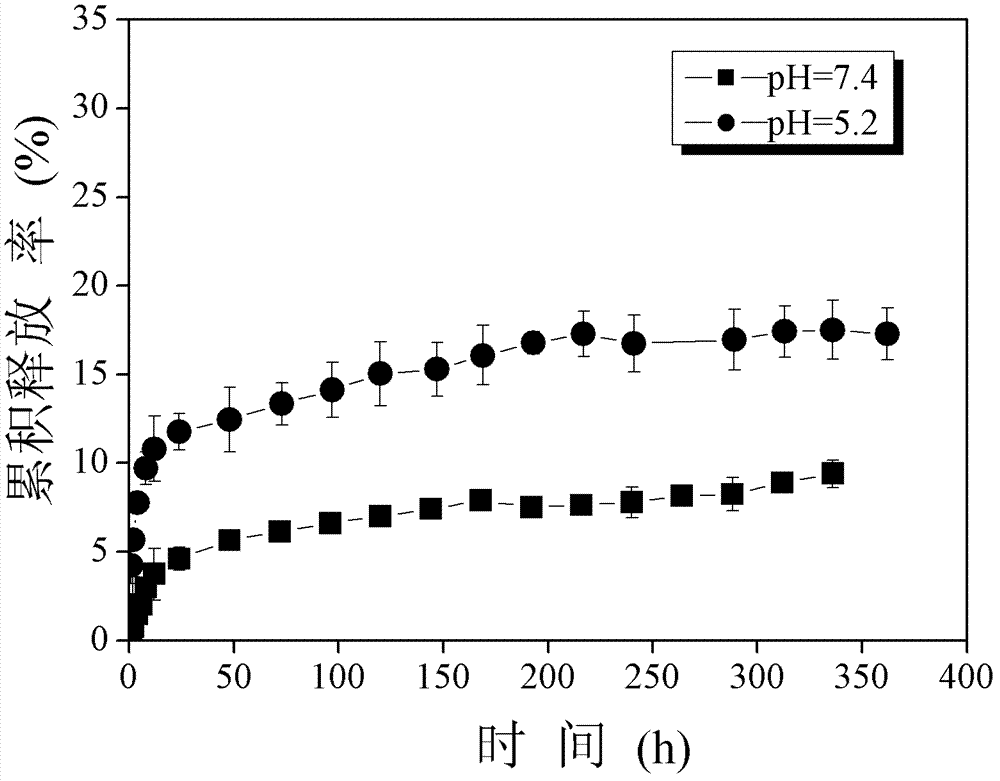
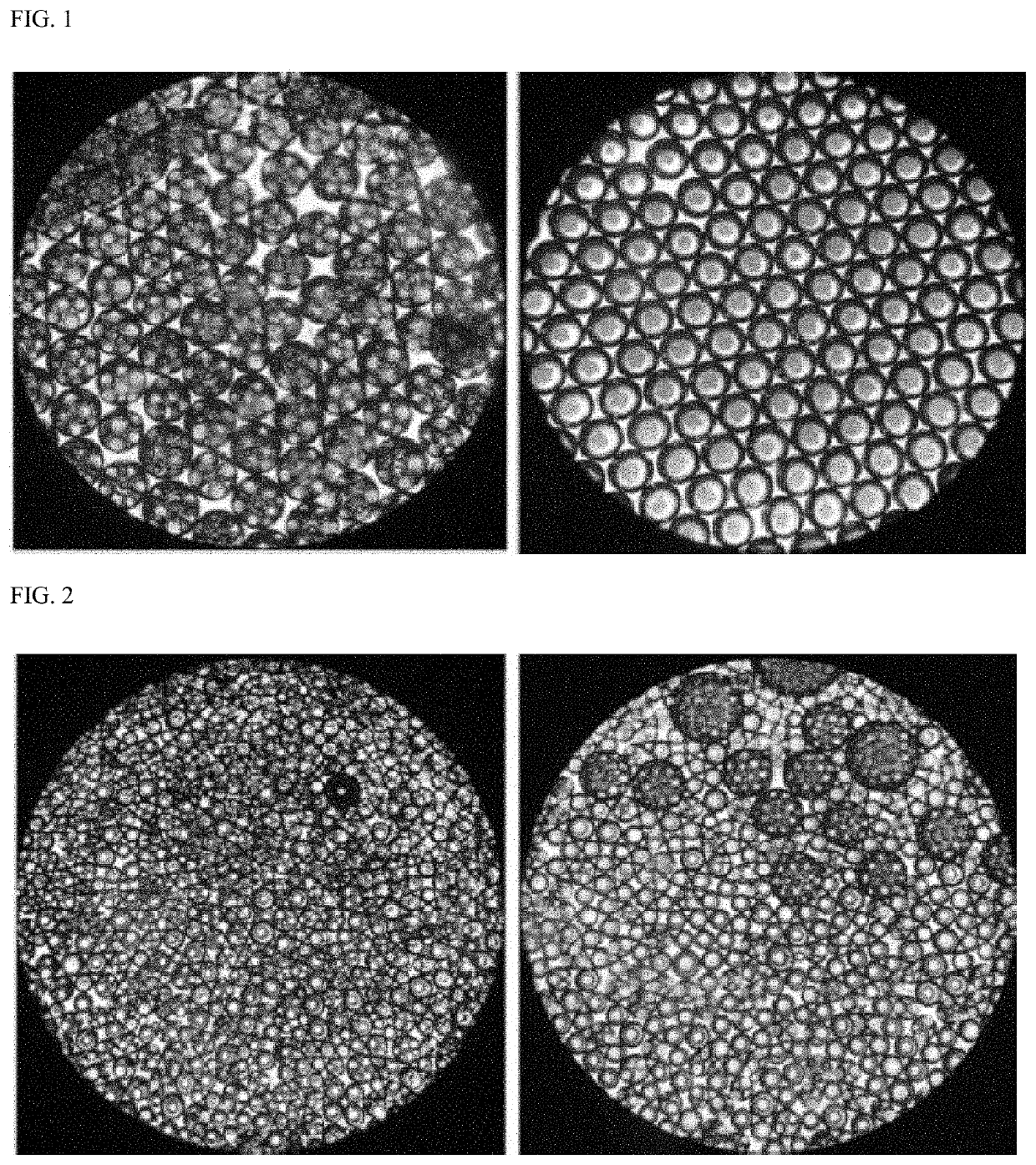
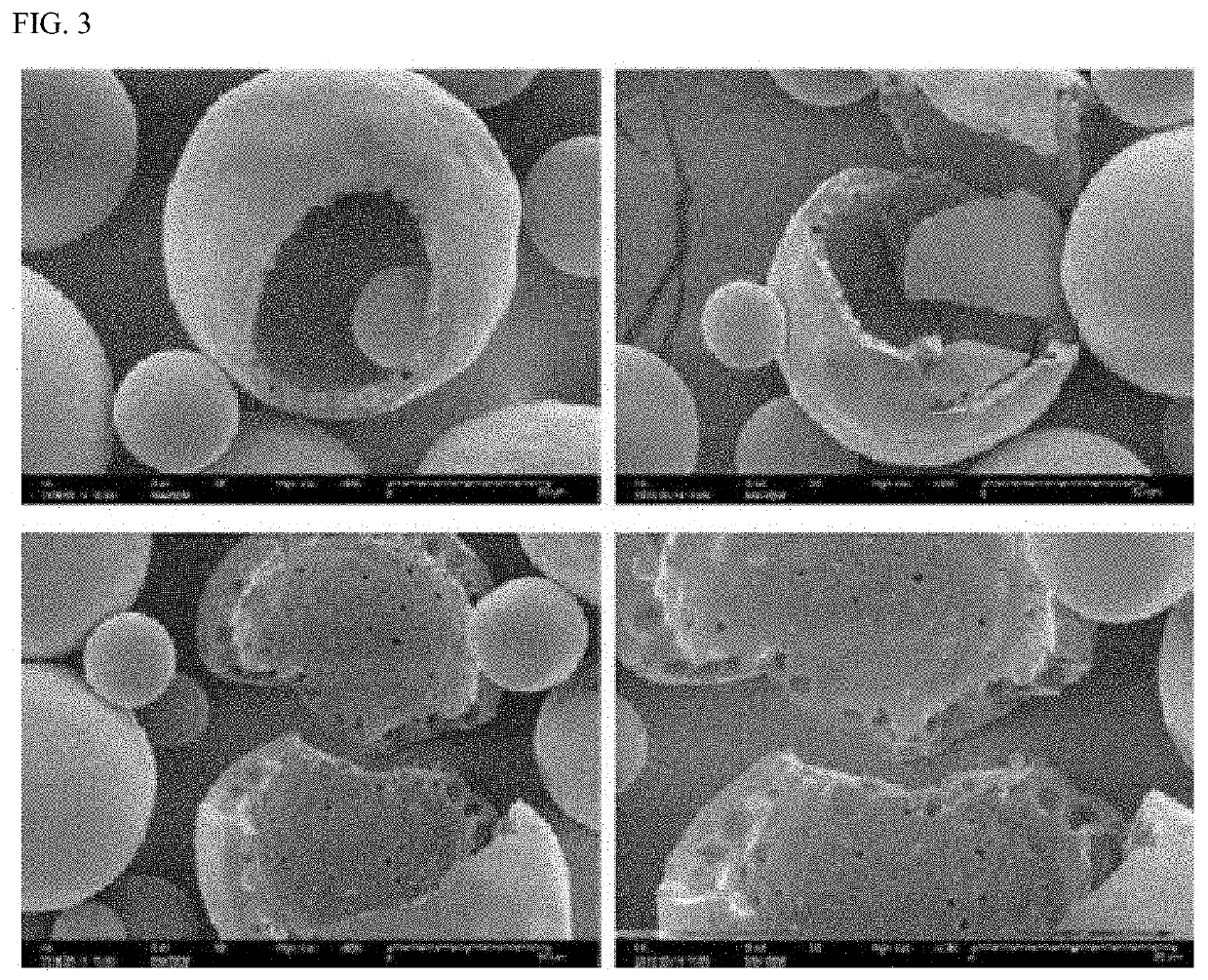
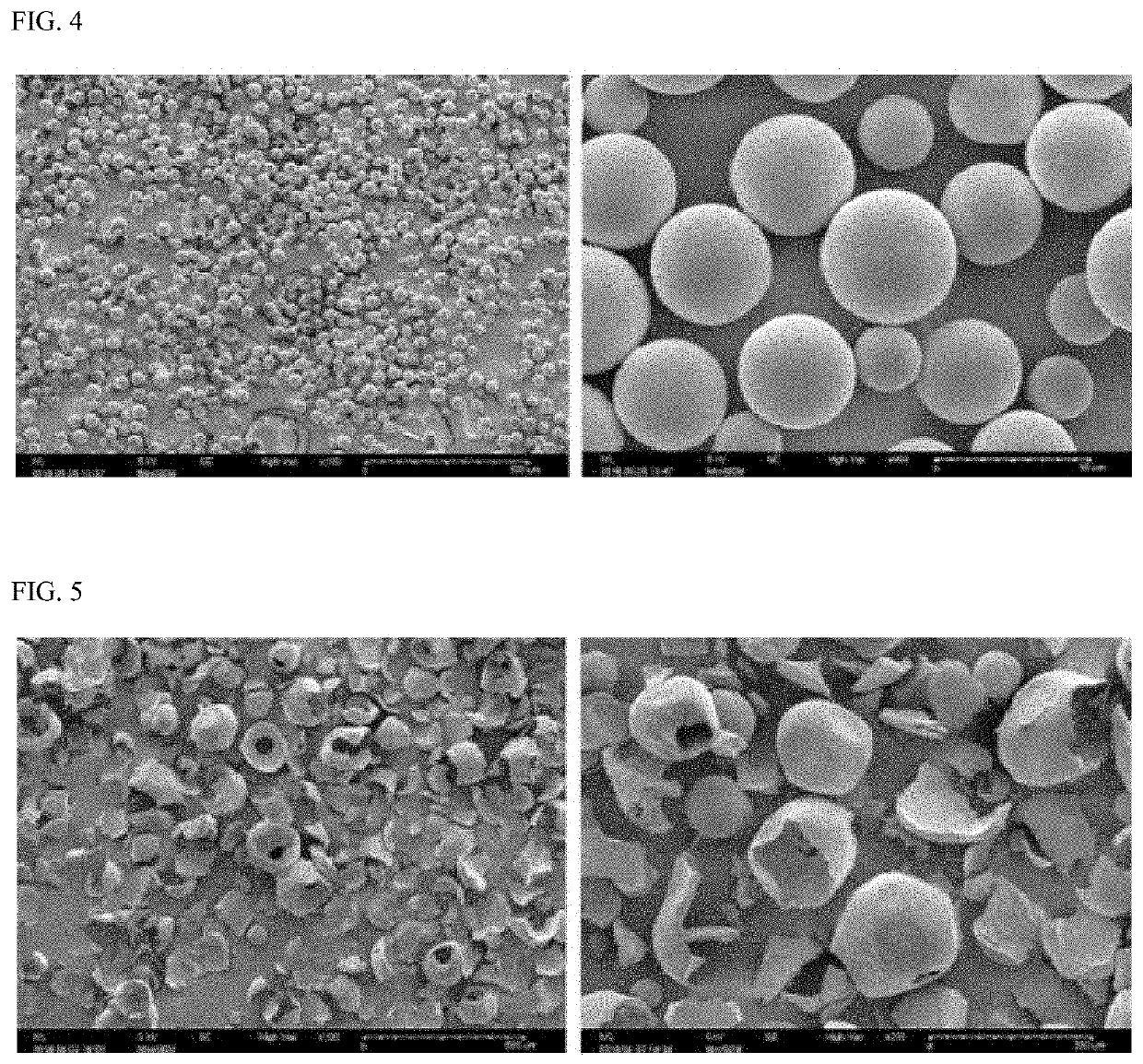
Composition for subcutaneous injection, containing deoxycholic acid, and preparation method therefor
PendingUS20210161917A1Avoid destructionGood effectPowder deliveryOrganic active ingredientsSurgical removalSingle injection
The present invention relates to a composition for subcutaneous injection, containing deoxycholic acid, and a preparation method therefor. The present invention relates to a composition for non-surgical removal of localized fat deposits, the composition being micro-particles, which comprise deoxycholic acid and a biodegradable polymer, wherein the microparticles are formed such that deoxycholic acid is evenly distributed in a spherical biodegradable polymer. According to the present invention, a lipolysis effect lasts for 1 to 3 months from a single injection, a phenomenon in which surrounding tissues are destroyed during administration is prevented, and a drug release effect can be maintained at an effective amount of adipolysis concentration.
Owner:INVENTAGE LAB INC
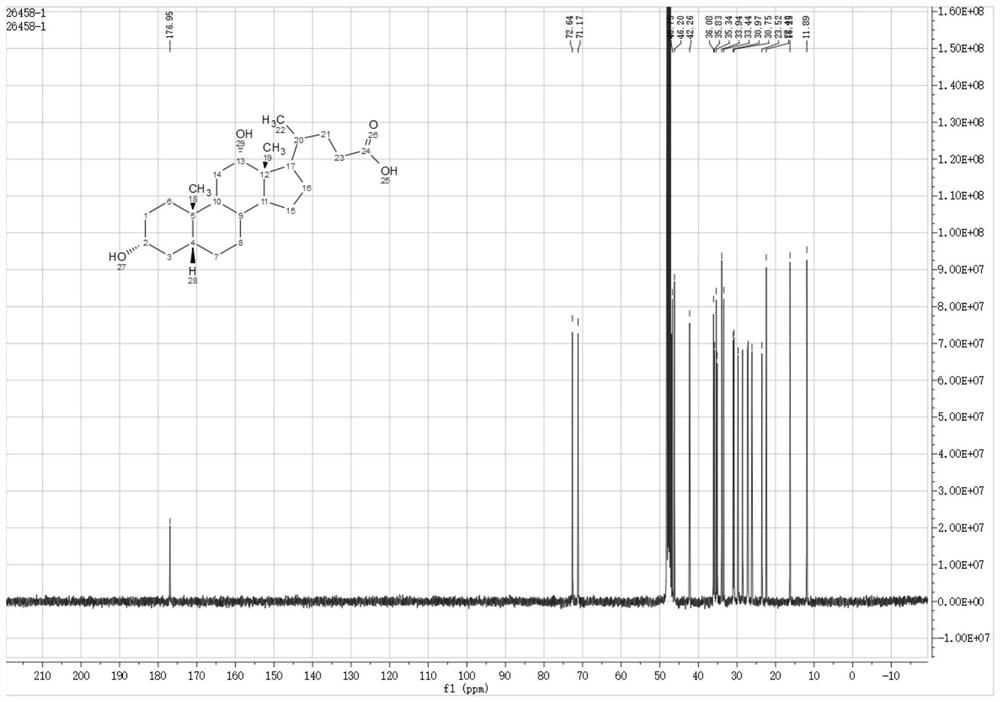
Preparation of deoxycholic acid
The present invention relates to new and improved processes for the preparation of deoxycholic acid (DCA) and pharmaceutically acceptable salts thereof, as well as to DCA and pharmaceutically acceptable salts thereof, the carbon atoms of which are derived solely from plant sources.
Owner:CRYSTAL PHARMA SA
A kind of purification method of sodium glycochenodeoxycholate
Owner:YAOPU SHANGHAI PHARMA TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com