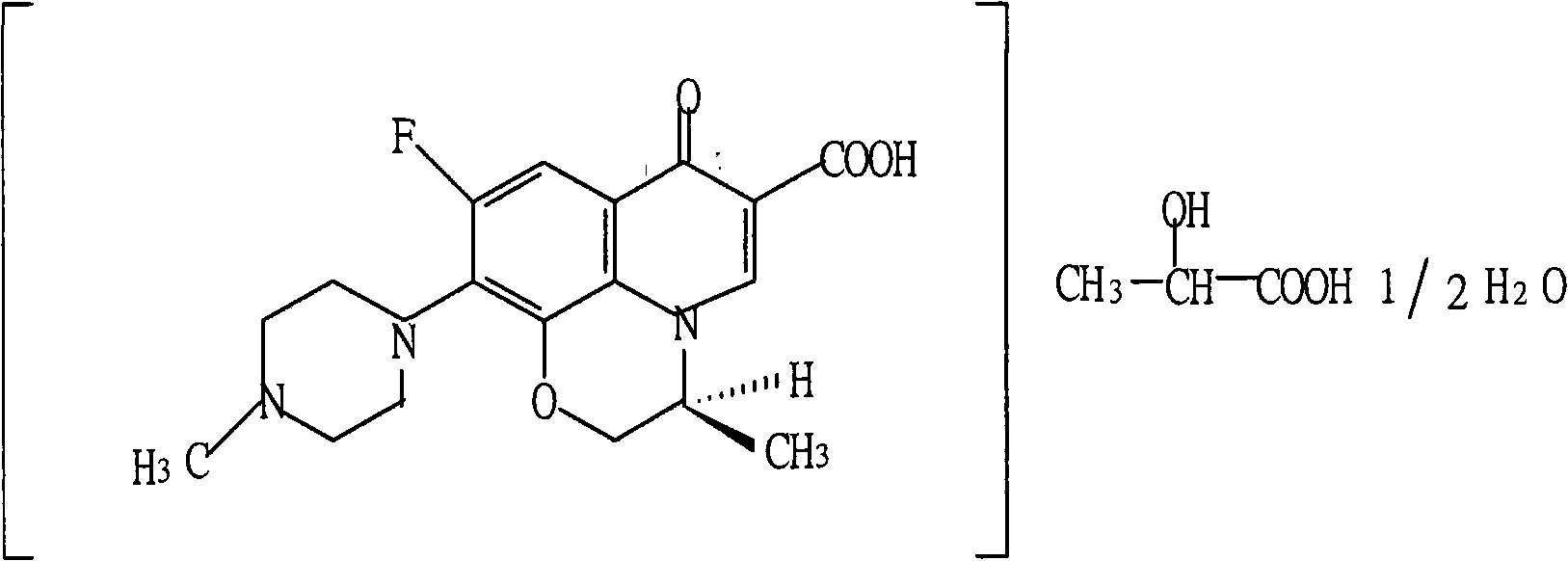
Levofloxacin lactate liposome sodium chloride injection and preparation method thereof
A technology of levofloxacin lactate and levofloxacin lactate is applied in the field of medicine and can solve the problems of unqualified clarity and insoluble particles of levofloxacin lactate injection, and the color of the solution turns yellow, so as to improve the therapeutic index of the drug, stabilize the product quality, and reduce the side effects of the drug. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
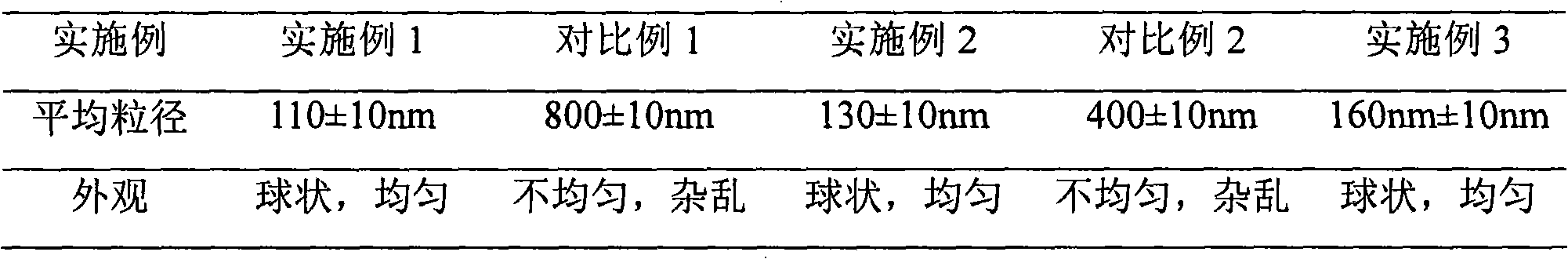
Embodiment 1
[0033] Example 1 Preparation of Levofloxacin Lactate Liposomal Sodium Chloride Injection
[0034] Prescription (100 bottles): Levofloxacin Lactate 10g
[0035] Soy Lecithin 60g
[0036]Cholesterol 5g
[0037] Sodium deoxycholate 15g
[0038] Ascorbyl Palmitate 10g
[0039] Sodium chloride 90g
[0040] Preparation Process
[0041] (1) 60g soybean lecithin, 5g cholesterol, 15g sodium deoxycholate and 10g ascorbyl palmitate are dissolved in the ethanol of 500ml;
[0042] (2) levofloxacin lactate 10g and sodium chloride 90g are dissolved in the potassium dihydrogen phosphate-dipotassium hydrogen phosphate buffer solution of 8000mlpH value 5.0;
[0043] (3) Mix the two and stir to form a W / O emulsion, heat and stir to evaporate, when the mixture reaches a viscous state, add 2000ml of phosphate buffer with a pH value of 5.0, continue to heat and stir to evaporate to remove residual ethanol, and ultrasonic for 30min , transferred to a high-speed homogeneous mixer, stirred a...
Embodiment 2
[0055] Example 2 Preparation of Levofloxacin Lactate Liposomal Sodium Chloride Injection
[0056] Prescription (100 bottles): Levofloxacin Lactate 20g
[0057] Phosphatidylglycerol Distearate 200g
[0058] Cholesterol 20g
[0059] Sodium deoxycholate 40g
[0060] Sodium bisulfite 20g
[0061] Sodium chloride 90g
[0062] Preparation Process
[0063] (1) Dissolve 200g phosphatidylglycerol distearate, 20g cholesterol, 40g sodium deoxycholate and 20g sodium bisulfite in isopropanol of 2000ml;
[0064] (2) levofloxacin lactate 20g and sodium chloride 90g are dissolved in 8000ml water;
[0065] (3) Mix the two, stir to form a W / O emulsion, heat and stir to evaporate, when the mixture reaches a viscous state, add 2000ml of acetic acid-sodium acetate buffer solution with a pH value of 4.6, continue to heat and stir to evaporate to remove residual isopropyl Alcohol, ultrasonic 30min, transferred to a high-speed homogeneous mixer, stirred homogeneously for 30min, to obtain l...
Embodiment 3
[0077] Example 3 Preparation of Levofloxacin Lactate Liposomal Sodium Chloride Injection
[0078] Prescription (100 bottles): Levofloxacin Lactate 30g
[0079] Egg Yolk Phosphatidylserine 400g
[0080] Cholesterol 30g
[0081] Sodium deoxycholate 25g
[0082] Vitamin E 15g
[0083] Sodium chloride 90g
[0084] Preparation Process
[0085] (1) 400g egg yolk phosphatidylserine, 30g cholesterol, 25g sodium deoxycholate and 15g vitamin E are dissolved in 2000ml of benzyl alcohol;
[0086] (2) levofloxacin lactate 30g and sodium chloride 90g are dissolved in the citric acid-sodium citrate buffer solution of 8000mlpH value 4.8;
[0087] (3) Mix the two and stir to form a W / O emulsion, heat and stir to evaporate, when the mixture reaches a viscous state, add 2000ml of water, continue to heat and stir to evaporate to remove residual benzyl alcohol, ultrasonic 30min, transfer to a high-speed uniform In a mass m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com