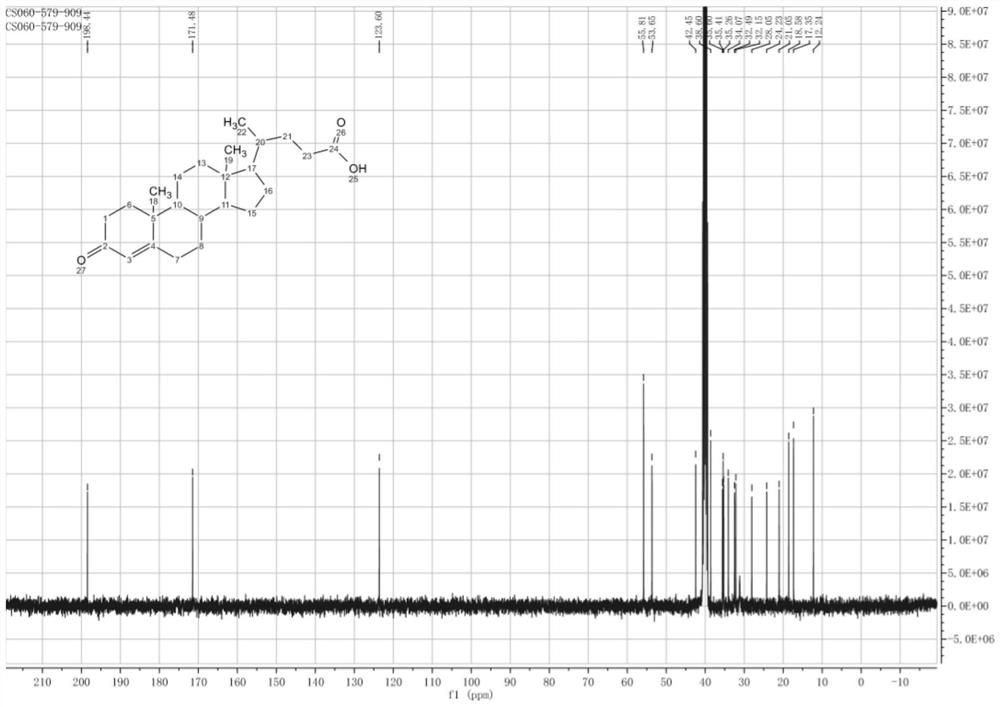
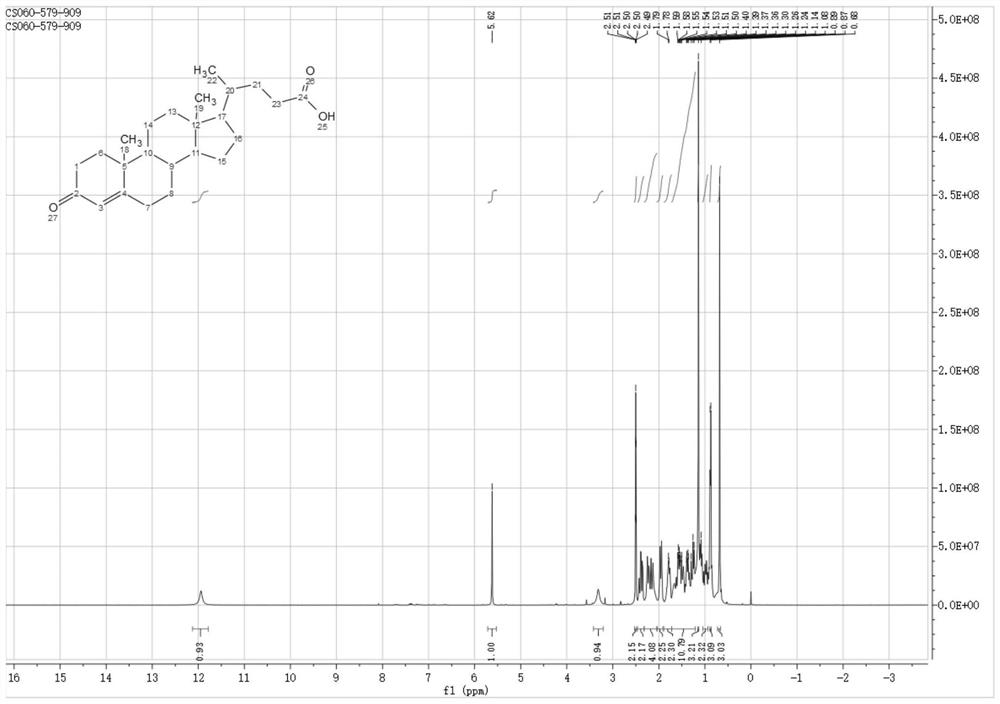
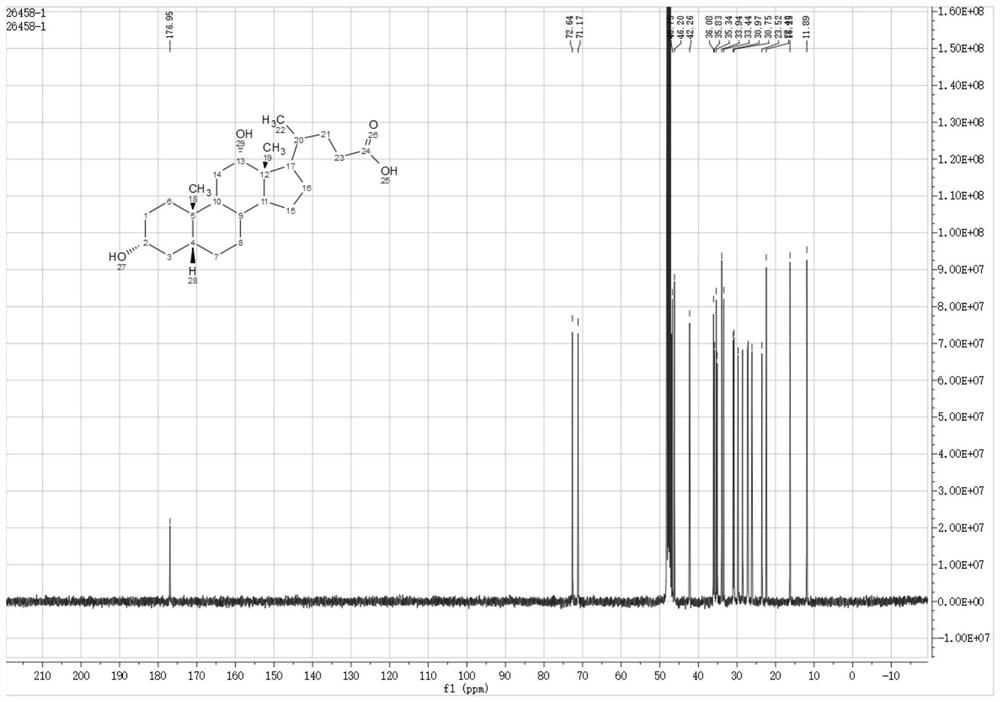
Method for preparing deoxycholic acid from phytosterol
A technology of deoxycholic acid and phytosterols, which is applied in the field of preparation of deoxycholic acid compounds in a combination of biological fermentation and chemical synthesis, can solve the problems of unsuitability for industrial production, long reaction steps, complicated process, etc. Less, mild reaction conditions, high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] The preparation of embodiment 1 compound 1
[0068] 1. Original strain transformation
[0069] 1 strain
[0070] Mycobacterium sp. NRRL B-3805
[0071] 2 conversion process
[0072] 2.1 Seed culture
[0073] First-class seeds: yeast extract powder 10g / L, glucose 15g / L, sodium nitrate 5.4g / L, diammonium hydrogen phosphate 0.06%, pH=7.5, 100ml medium into 500ml shaker flask, sterilized at 121°C for 30 minute. Inoculate from the slant after cooling, culture at 200rpm, 30°C for 48 hours.
[0074] Secondary seeds: yeast extract powder 10g / L, glucose 15g / L, sodium nitrate 5.4g / L, diammonium hydrogen phosphate 0.06%, pH=7.5, put 500ml medium into 2000ml shaker flask, sterilize at 121℃ for 30 minute. After cooling, inoculate from the primary shaker flask with an inoculum size of 10%, culture at 200 rpm, and 30°C for 48 hours.
[0075] 2.2 Conversion
[0076] 10 liter fermenter, 7 liters of sample, medium: stigmasterol 20g / L, soybean oil 160g / L, corn steep liquor 60g / L,...
Embodiment 2
[0099] The preparation of embodiment 2 compound 2
[0100] 1. Seed cultivation
[0101] (1) Incline cultivation
[0102] Adopt Aspergillus ochraceus ATCC 18500 as the production strain, inoculate the preserved bacterial strain by streaking on the slant, and cultivate it for 6-7 days at 30°C. The slant medium is potato medium: potato pieces 200g / L (boiled for 30 minutes , four layers of gauze filter to get the filtrate), glucose 20g / L, agar 20g / L, pH6.5.
[0103] (2) Seed cultivation
[0104] Preparation of spore suspension: Take a fresh slant that has been cultured for 6-7 days, wash the spores of the slant with 0.05% Tween-80 sterile water to make a spore suspension, and count the spore concentration under the microscope to be 2 to 3×10 7 a / ml;
[0105] Shake flask seed culture: inoculum size 10%, culture at 30°C, 180rpm for 36-48h.
[0106] The composition of the seed medium is as follows: corn steep liquor 10g / L, glucose 30g / L; pH value 7.2±0.2.
[0107] Sterilize by ...
Embodiment 3
[0117] The preparation of embodiment 3 compound 2 '
[0118] 1 strain
[0119] The name of the strain: Mycobacterium smegmatis NK-XHX-118 is preserved in the China Center for Type Culture Collection, and the preservation number is CCTCC NO: M2013544.
[0120] 2 conversion process
[0121] 2.1 Seed culture
[0122] First-class seeds: yeast extract powder 10g / L, glucose 15g / L, sodium nitrate 5.4g / L, diammonium hydrogen phosphate 0.06%, pH=7.5, 100ml medium into 500ml shaker flask, sterilized at 121°C for 30 minute. Inoculate from the slant after cooling, culture at 200rpm, 30°C for 48 hours.
[0123] Secondary seeds: yeast extract powder 10g / L, glucose 15g / L, sodium nitrate 5.4g / L, diammonium hydrogen phosphate 0.06%, pH=7.5, put 500ml medium into 2000ml shaker flask, sterilize at 121℃ for 30 minute. After cooling, inoculate from the primary shaker flask with an inoculum size of 10%, culture at 200 rpm, and 30°C for 48 hours.
[0124] 2.2 Conversion
[0125] 10 liter fer...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com