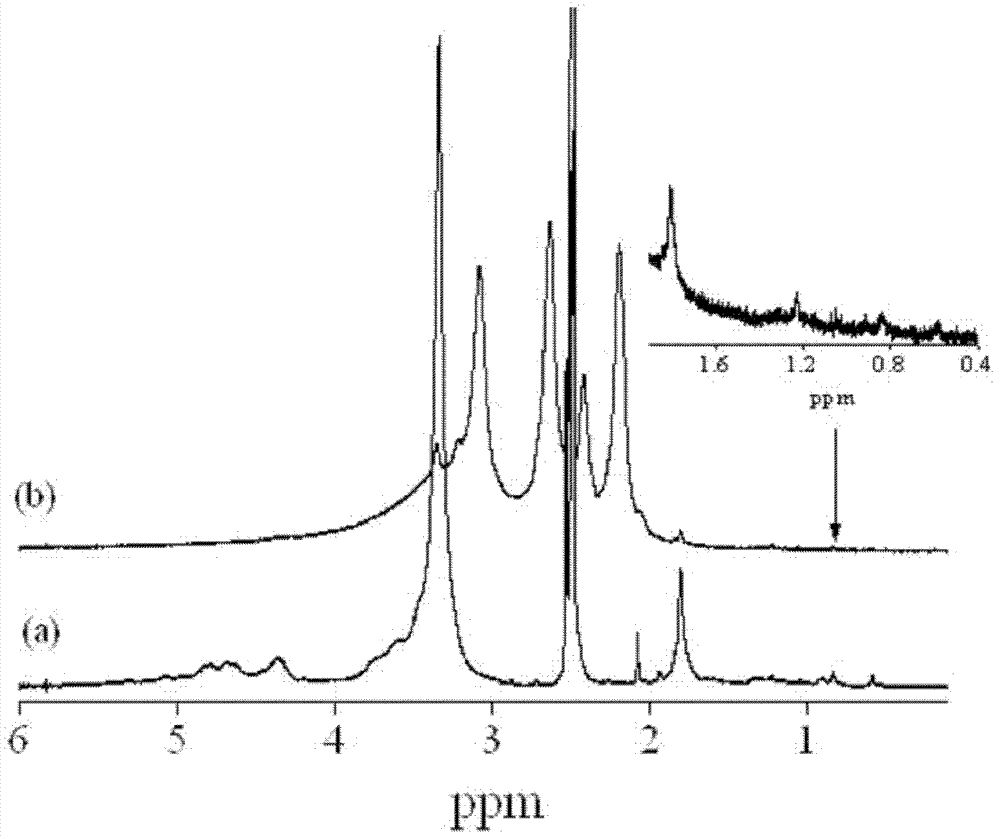
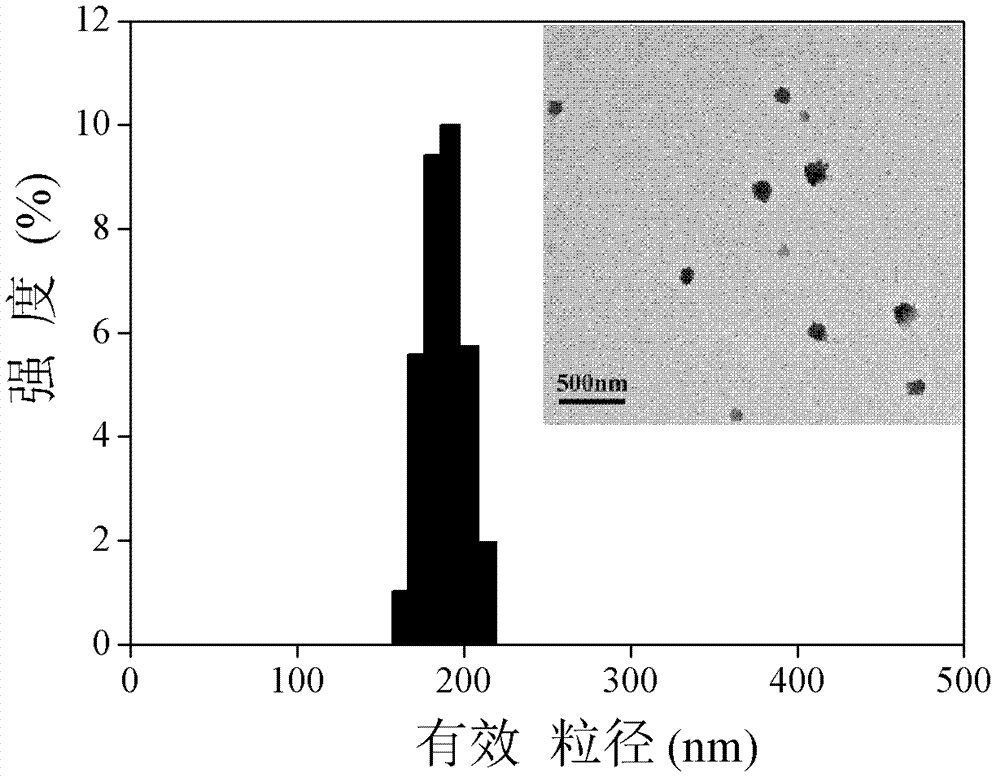
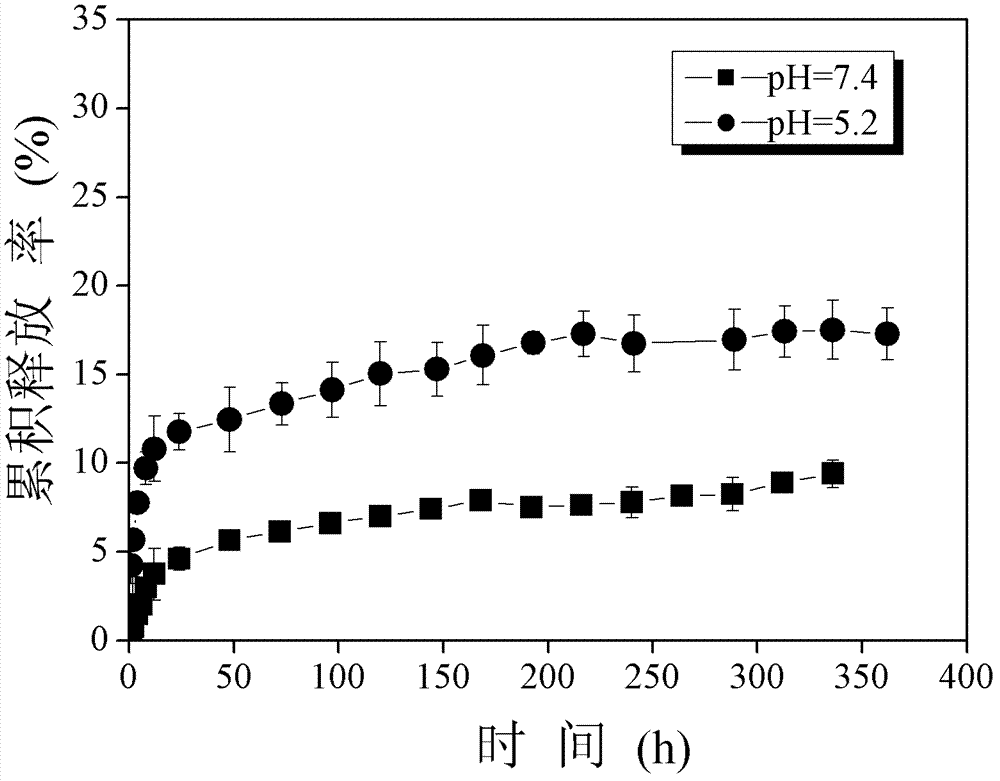
Amphipathic chitosan derivative and preparation method and application thereof
A technology of chitosan derivatives and chitosan, which is used in drug combinations, pharmaceutical formulations, organic active ingredients, etc., can solve problems such as few studies on natural polymer modification, achieve potential application value, and mild preparation reaction conditions. , a rich source of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of PAMAM-Cs-DCA
[0045] (1) Synthesis of 6-azido-N-amino-chitosan-g-deoxycholic acid graft polymer:
[0046] a Under the protection of nitrogen, 3.0g chitosan (weight average molecular weight is 10000, deacetylation degree is 70%) is dissolved in 60mLN, in the mixed solvent of N-dimethylformamide and water (95:5, v / v ), add 9.0g phthalic anhydride, stir and react at 120°C for 8 hours, after the reaction, cool to 25°C, precipitate with ice water, filter, wash the resulting product three times with methanol, and dry it in vacuum to obtain N- Phthalamido-chitosan, yield: 80%.
[0047] bUnder the protection of nitrogen, dissolve 1.0g N-phthalamido-chitosan in 100mL N-methyl-pyrrolidone, stir to dissolve, and add 6.05g N-bromosuccinimide in an ice-water bath and 10.3g triphenylphosphine, heated up to 70°C and reacted with magnetic stirring under nitrogen protection for 6 hours (rotating speed was 600 rpm). After the reaction, the product was centrifu...
Embodiment 2
[0056] Example 2 Preparation of PAMAM-Cs-DCA
[0057] (1) Synthesis of 6-azido-N-amino-chitosan-g-deoxycholic acid graft polymer:
[0058] a Under nitrogen protection, 3.0g chitosan (weight average molecular weight is 14000, deacetylation degree is 90%) is dissolved in 50mL N, in the mixed solvent of N-dimethylformamide and water (90:10, v / v), add 10g of phthalic anhydride, stir and react at 100°C for 10 hours, after the reaction, cool to room temperature at 25°C, precipitate with ice water, filter, wash the resulting product with methanol three times, and dry it under vacuum to obtain N - Phthalamido-chitosan, yield: 85%.
[0059] bUnder the protection of nitrogen, dissolve 1.0g N-phthalamido-chitosan in 50mL N-methyl-pyrrolidone, stir to dissolve, and add 7.26g N-bromosuccinimide in an ice-water bath And 12.4g triphenylphosphine, be warming up to 80 ℃ and under nitrogen protection magnetic stirring reaction 4 hours (rotating speed is 1200 rev / min), after reaction finishes...
Embodiment 3
[0068] Example 3 Preparation of PAMAM-Cs-DCA
[0069] (1) Synthesis of 6-azido-N-amino-chitosan-g-deoxycholic acid graft polymer:
[0070] a Under nitrogen protection, 3.2g chitosan (weight average molecular weight is 12000, deacetylation degree is 80%) is dissolved in 60mL N, in the mixed solvent of N-dimethylformamide and water (14:1, v / v), add 10g of phthalic anhydride, stir and react at 110°C for 9 hours, after the reaction is over, cool to 30°C, precipitate with ice water, filter, wash the resulting product with methanol three times, and obtain N- Phthalamido-chitosan, yield: 83%.
[0071] bUnder the protection of nitrogen, dissolve 1.0g N-phthalamido-chitosan in 75mL N-methyl-pyrrolidone, stir to dissolve, and add 6.50g N-bromosuccinimide in an ice-water bath And 11.0g triphenylphosphine, be warming up to 75 ℃ and under nitrogen protection magnetic stirring reaction 5 hours (rotating speed is 900 rev / min), after reaction finishes, the product is centrifuged, filtered,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com