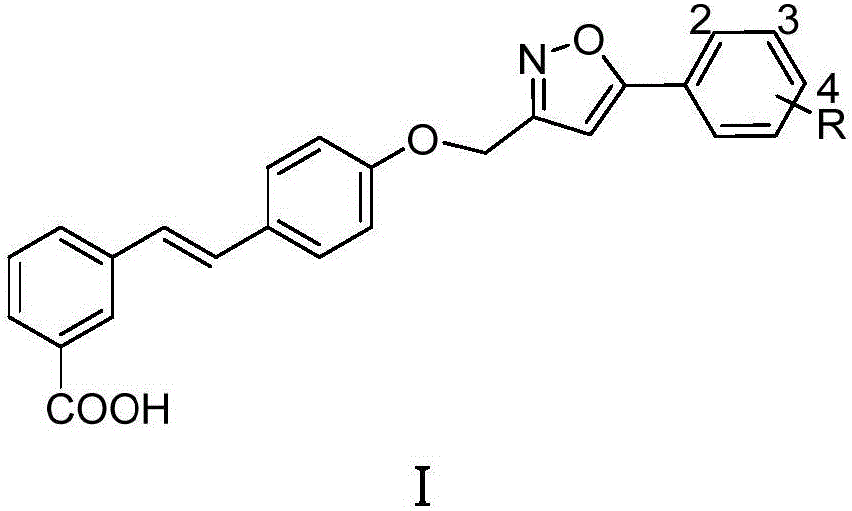
5-phenyl isoxazole-containing stilbenoid compound and preparation method thereof
A technology for phenylisoxazole and compounds, which is applied in the field of compound preparation and can solve problems such as poor drug-like properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
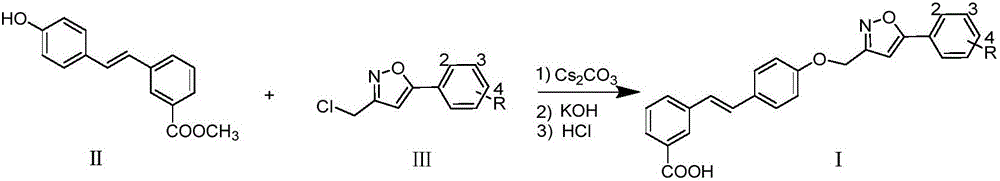
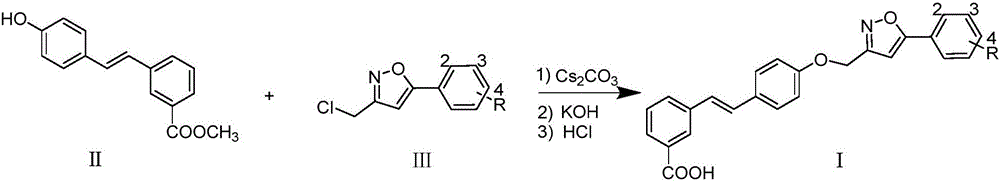
[0014] Embodiment 1: the synthesis of compound Ia (R=H)
[0015]
[0016] Compound II (1mmol), IIIa (R=H, 1mmol) and cesium carbonate (2mmol) were heated to 85°C in DMSO (5mL) solvent and kept at this temperature for 8 hours, and 20% hydrogen was added after reducing the reaction temperature to 60°C Potassium oxide solution (2 mL), reacted and stirred at 60° C. for 4 hours. Cool the reaction night to room temperature, acidify with hydrochloric acid and dilute with water to obtain a large amount of precipitate, filter and recrystallize with methanol to obtain compound Ia (R=H); Yellow solid; Yield: 42%; 1 H NMR (DMSO-d 6 ,400MHz)δ:13.15(brs,1H),8.12(s,1H),7.90(dd,J 1 =8.0Hz,J 2 =1.8Hz, 2H), 7.80(t, J=7.5Hz, 2H), 7.62(d, J=8.8Hz, 2H), 7.52~7.58(m, 3H), 7.47(t, J=7.7Hz, 1H ),7.29(d,J=16.4Hz,1H),7.22(d,J=16.4Hz,1H),7.20(s,1H),7.10(d,J=8.8Hz,2H),5.29(s,2H ); 13 CNMR (DMSO-d 6 ,100MHz)δ:170.0,168.2,161.8,158.1,138.0,131.1,130.8,130.3,129.8(2C),129.3,129.2,128.5(2C),128.4,1...
Embodiment 2
[0017] Embodiment 2: the synthesis of compound Ib (R=2-Cl)
[0018] Substitute IIIb (R=2-Cl) for IIIa (R=H), and obtain it by referring to the synthesis method of Ia in Example 1. Yellowsolid; Yield: 51%; 1 H NMR (DMSO-d 6 ,400MHz)δ:12.98(brs,1H),8.13(s,1H),7.82~7.88(m,2H),7.79(d,J=8.1Hz,1H),7.62(d,J=8.6Hz,2H ),7.49~7.54(m,1H),7.35(d,J=8.1Hz,2H),7.47(t,J=7.7Hz,1H),7.28(d,J=16.5Hz,1H),7.23(d ,J=16.5Hz,1H),7.10(d,J=8.6Hz,2H),7.09(s,1H),5.27(s,2H),2.37(s,3H);170.2,166.7,161.7,158.2, HRMS(ESI) calcd for C 25 h 17 ClNO 4 [M-H] - 430.0846, found 430.0831.
Embodiment 3
[0019] Embodiment 3: the synthesis of compound Ic (R=2-OMe)
[0020] Substitute IIIc (R=2-OMe) for IIIa (R=H), and obtain it by referring to the synthesis method of Ia in Example 1. Yellowsolid; Yield: 47%; 1 H NMR (DMSO-d 6 ,400MHz)δ:13.05(brs,1H),8.13(s,1H),7.79~7.83(m,2H),7.62(d,J=8.8Hz,2H),7.46~7.52(m,3H),7.26 (d, J=16.4Hz, 1H), 7.20(d, J=16.4Hz, 1H), 7.10~7.14(m, 2H), 6.99(s, 1H), 6.79(d, J=8.8Hz, 2H) ,5.29(s,2H),3.96(s,3H); 13 C NMR (DMSO-d 6 ,100MHz)δ:167.8,166.2,161.5,158.2,138.4,138.2,131.7,130.7,130.5,130.0,129.4,129.4,128.6(2C),128.2,127.4,127.2,126.1,124.6,121.6(2C) , 103.6, 61.8, 56.3; HRMS (ESI) calcd for C 26 h 20 NO 5 [M-H] - 426.1341, found 426.1376.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com