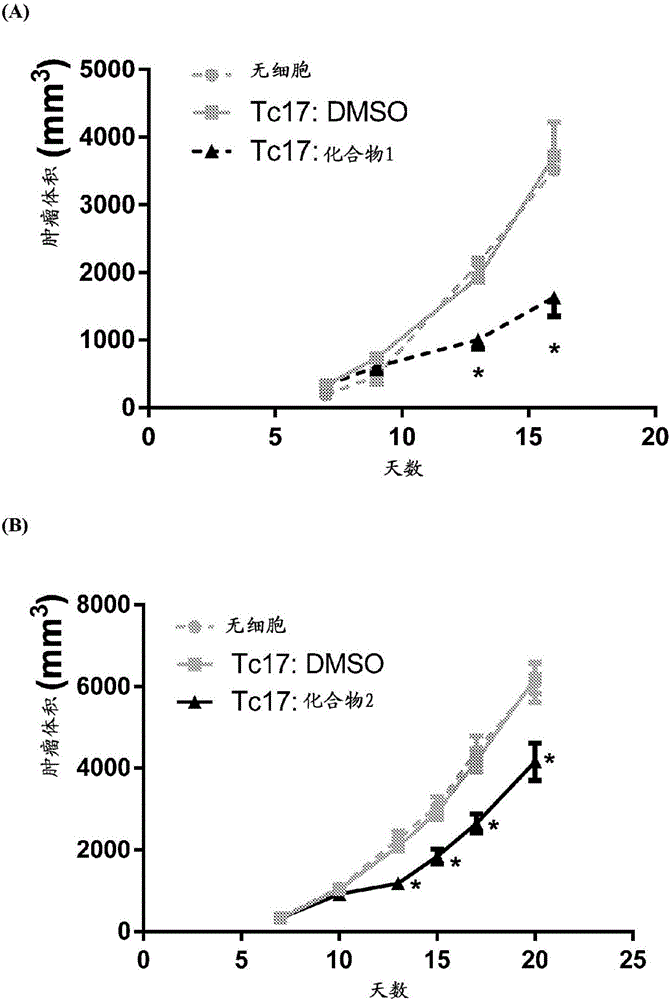
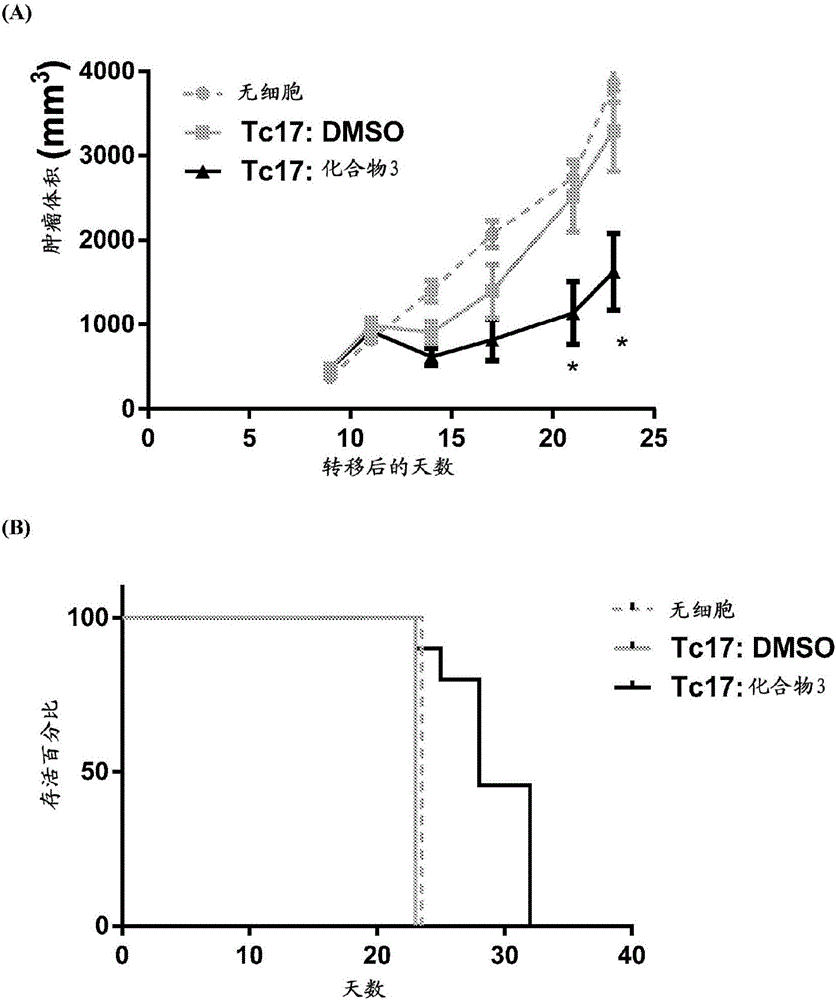
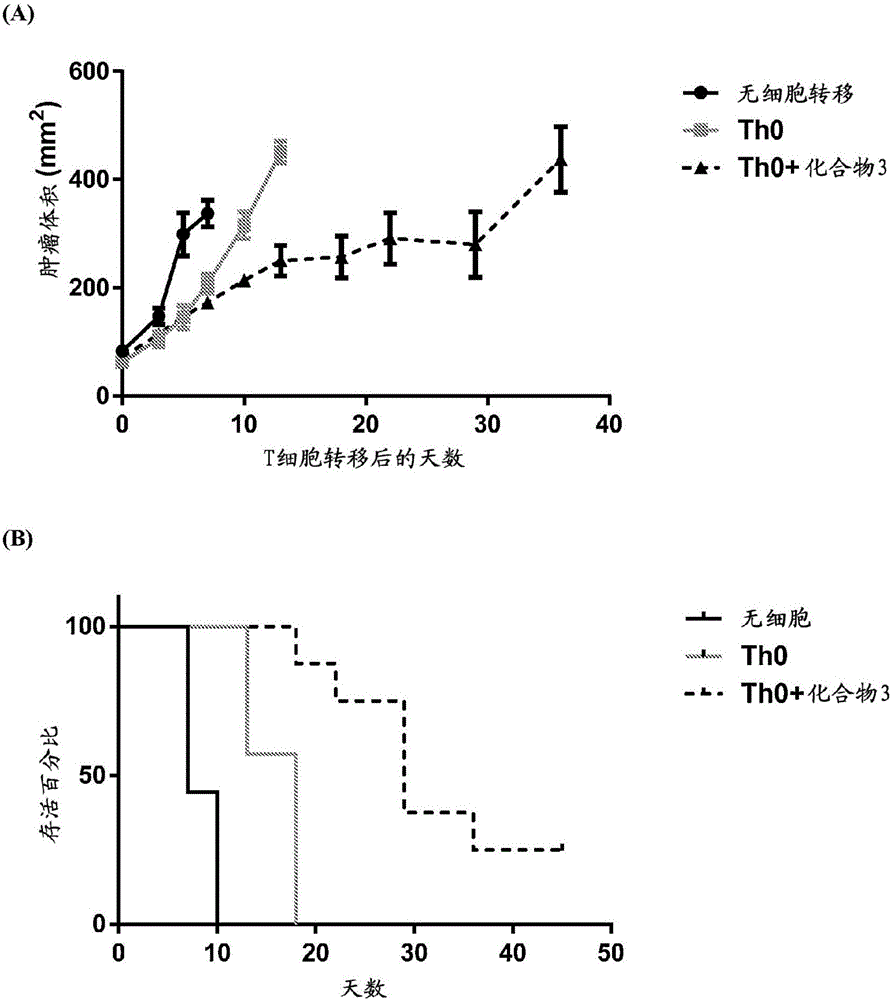
Adoptive cellular therapy using an agonist of retinoic acid receptor-related orphan receptor gamma & related therapeutic methods
A gamma agonist, cell technology, applied in animal cells, vertebrate cells, genetically modified cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0562] Example 1 --(S)-6-((2-Chloro-6-fluorophenoxy)methyl)-2-methyl-4-((3-(trifluoromethyl)phenyl)sulfonyl)-3 , Synthesis of 4-dihydro-2H-benzo[b][1,4]]oxazine
[0563]
[0564] Part I--Synthesis of methyl (S)-4-((1-methoxy-1-oxopropan-2-yl)oxy)-3-nitrobenzoate
[0565]
[0566] Methyl-4-hydroxy-3-nitrobenzoate (3g, 13.76mmol), methyl-(R)-lactate (1.433g, 13.8mmol) and triphenylphosphine (4.33g, 16.5 mmol) was suspended in dichloromethane (36 mL), and diisopropyl azodicarboxylate (3.25 mL, 16.51 mmol) was added dropwise. The reaction mixture was stirred for 1 h at room temperature, then the crude material was washed with water and dried (Na 2 SO 4 ) and concentrated to give a residue. The residue was purified via MPLC elution with a gradient of ethyl acetate in hexanes, followed by a second MPLC purification elution with dichloromethane to afford (S)-4-((1 -Methoxy-1-oxopropan-2-yl)oxy)-3-nitrobenzoate (2.56 g, 66%). 1 H-NMR (400MHz, DMSO-d 6 )8.37(s,1H),8.12(d...
Embodiment 2
[0579] Example 2 (S,E)-3-(6-(2-(2-chloro-6-fluorophenyl)prop-1-en-1-yl)-4-((3-(trifluoromethyl)phenyl Synthesis of )sulfonyl)-3,4-dihydro-2H-benzo[b][1,4]oxazin-2-yl)propionic acid
[0580]
[0581] Part I--Synthesis of (R)-2-hydroxyglutaric acid dimethyl ester
[0582]
[0583] To a mixture of (2R)-5-oxotetrahydro-2-furoic acid (25 g, 192 mmol) in methanol (300 mL) was added concentrated hydrogen chloride (0.5 mL), and the mixture was refluxed overnight. The reaction mixture was then cooled to ambient temperature, solid sodium bicarbonate was added, and the resulting mixture was slurried for 20 minutes. Then, the mixture was filtered and concentrated to give (R)-dimethyl 2-hydroxyglutarate (34.7 g, 100%).
[0584] Part II--Synthesis of dimethyl (S)-2-(4-bromo-2-nitrophenoxy)glutarate
[0585]
[0586] (R)-Dimethyl 2-hydroxyglutarate (33.8g, 192mmol), 4-bromo-2-nitrophenol (50.2g, 230mmol) and triphenylphosphine with activated molecular sieves were added at 0°C ...
Embodiment 3
[0610] Example 3 --(S,E)-3-(6-(2-Chloro-6-(trifluoromethyl)styryl)-4-((3-(trifluoromethyl)phenyl)sulfonyl)- Synthesis of 3,4-dihydro-2H-benzo[b][1,4]oxazin-2-yl)propionic acid
[0611]
[0612] Part I--Synthesis of 1-chloro-2-ethynyl-3-(trifluoromethyl)benzene
[0613]
[0614] To a solution of 2-chloro-6-(trifluoromethyl)benzaldehyde (10.0 g, 47.9 mmol) in methanol (100 mL) was added dimethyl(diazomethyl)phosphonate (11.05 g ,57.5mmol). The reaction mixture was cooled to 0 °C and potassium carbonate (16.6 g, 119 mmol) was added. The reaction mixture was stirred overnight at room temperature. Then, the resulting crude mixture was diluted with ether, washed with water, washed with brine, dried (MgSO 4 ) and concentrated to give 1-chloro-2-ethynyl-3-(trifluoromethyl)benzene (9.17 g, 93%).
[0615] Part II--(E)-2-(2-Chloro-6-(trifluoromethyl)styryl)-4,4,5,5-tetramethyl-1,3,2-dioxane Synthesis of pentaborane
[0616]
[0617] [2,3-bis(1-adamantyl)imidazolin-2-yl-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com