Ruminant clostridial disease quadruple inactivated vaccine and preparation method thereof
A technology of inactivated vaccines and ruminants, which can be applied to antibacterial drugs, bacterial antigen components, etc., and can solve the problem of low success rate of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] ——Preparation of quadruple inactivated vaccine against clostridial disease in ruminants
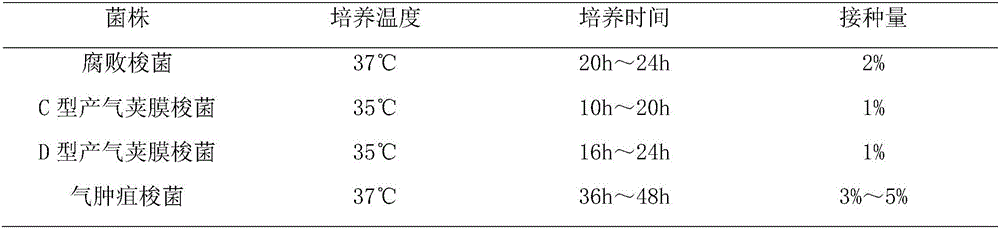
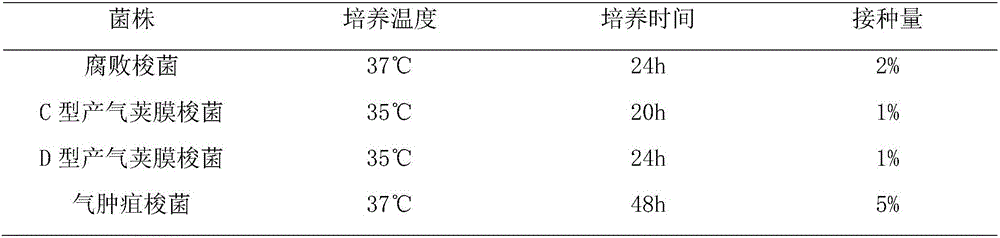
[0055] 1. Strains of Clostridium emphysema C54-2, Clostridium putrefaction C55-1, C-type Clostridium perfringens C59-2 and D-type Clostridium perfringens C60-2 (see Please see China Veterinary Drug Supervision Institute, China Veterinary Microbiological Strain Preservation Management Center edited, Chinese Veterinary Strain Catalog (Second Edition), China Agricultural Science and Technology Press, 2002, p40, 52, 48, 50) strains are all purchased From the Bacteria and Virus Preservation Center of the China Veterinary Drug Control Institute, it is used as a production strain.
[0056] 2. Culture medium Clostridium putrefaction uses trypsin to digest beef broth, Clostridium perfringens uses meat liver and stomach enzyme digestion soup (or cod liver and stomach enzyme digestion soup), Clostridium emphysema uses anaerobic meat liver soup, All were purchased from Rongcheng Yulin Biologi...
Embodiment 2
[0071] —— Vaccine Safety Trials
[0072] (1) Three components of Clostridium putrefaction, Clostridium perfringens type C and Clostridium perfringens type D
[0073] Inoculate 5 2kg rabbits with the prepared vaccine, each subcutaneously inject 2ml (equivalent to 5 sheep immunization doses), observe for 8 days, and all 5 rabbits are healthy and alive.
[0074] (2) Emphysema part
[0075] 2 guinea pigs with a body weight of 400 g were injected subcutaneously with 2 ml of vaccine (equivalent to 2 sheep immunization doses), observed for 8 days, and all 5 guinea pigs were healthy and alive.
[0076] The above results show that the quadruple inactivated vaccine for ruminant clostridial disease prepared by the invention meets the standards of safety inspection.
Embodiment 3
[0078] - Vaccine efficacy test
[0079] (1) Parts of Clostridium putrefaciens, Clostridium perfringens type C and Clostridium perfringens type D
[0080] 1) Divide 30 rabbits with a body weight of 2kg into 6 groups, 5 in each group;
[0081] 2) Groups 1 to 3 were immunized by subcutaneous injection, and the immunization dose was 0.5ml, and groups 4 to 6 were not immunized and served as the control group;
[0082] 4) After 21 days of immunization, groups 1 and 4 were injected with 0.1ml (minimum lethal dose) of Clostridium putrefaction bacteria liquid, groups 2 and 5 were injected with 0.1ml (minimum lethal dose) of Clostridium perfringens toxin type C, 3, The 6 groups were injected with 0.1ml (minimum lethal dose) of D-type Clostridium perfringens poison.
[0083] 5) Observe for 5 days.
[0084] 6) The results determined that all the control rabbits died, and at least 4 immunized rabbits were qualified. The results are shown in Table 3.
[0085] Table 3 efficacy test resul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com