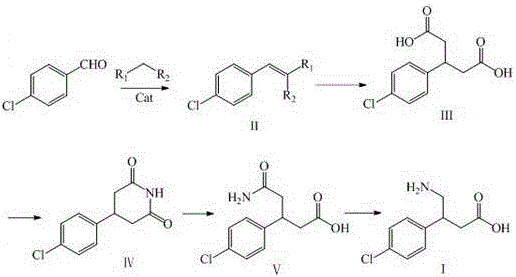
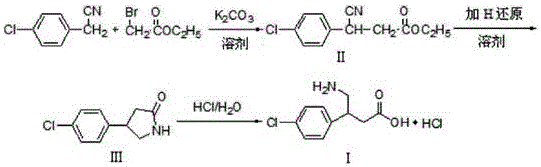
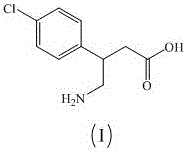
Green industrial production method of baclofen
A baclofen and green technology, applied in the field of producing GABA derivatives, can solve the problems of unsuitability for industrialized production, toxicity of raw materials and reagents, high cost of raw materials, etc., and achieve short production cycle, high reaction yield and high total yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] a. Preparation of Intermediate II:
[0049] 105g (0.75mol) of p-chlorobenzaldehyde, 400ml of deionized water, 137g (1.05mol) of ethyl acetoacetate, stirred at 15-20°C, slowly drop into 132ml of an aqueous solution of 13.2g (0.1mol) of diammonium hydrogen phosphate , the dropping was completed, kept stirring for 5 hours, filtered, washed the solid with appropriate amount of water, and dried under vacuum at 45-55°C for 4 hours to obtain white solid intermediate II, which was directly used in the next step.
[0050] b. Preparation of Intermediate III:
[0051] Intermediate Ⅱ, potassium hydroxide aqueous solution 420ml (30N), stirred and hydrolyzed at 85-90°C for 2h, TLC identification of the reaction end point (developing solvent: ethyl acetate-petroleum ether=2:3), after the reaction was completed, cooled to At room temperature, add 400ml of dichloromethane and 500ml of deionized water, stir for 20min, let stand to separate layers, discard the organic layer and recover i...
Embodiment 2
[0064] a. Preparation of Intermediate II:
[0065] 105g (0.75mol) of p-chlorobenzaldehyde, 137g (1.05mol) of ethyl acetoacetate, 63g (0.5mol) of melamine, ground at room temperature for 1 hour, after the reaction was completed, 700ml of ethyl acetate was added, stirred for 20 minutes, filtered, the filtrate was concentrated to dryness, solid Wash with an appropriate amount of water, and dry under vacuum at 45-55°C for 4 hours to obtain intermediate II as a white solid, which is directly used in the next step.
[0066] b. Preparation of Intermediate III:
[0067] Intermediate Ⅱ, potassium hydroxide aqueous solution 370ml (30N), stirred and hydrolyzed at 85-90°C for 2h, TLC identification of the reaction end point (developing agent: ethyl acetate-petroleum ether=2:3), after the reaction was completed, cooled to At room temperature, add 400ml of dichloromethane and 400ml of deionized water, stir for 20min, let stand to separate layers, discard the organic layer and recover it, u...
Embodiment 3
[0075] Ethyl cyanoacetate was used to replace step a of Example 1 to prepare intermediate II, and the rest of the steps were the same to obtain 32 g of baclofen with a yield of 78.5%, mp: 205.8-206.3°C, and HPLC content of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com