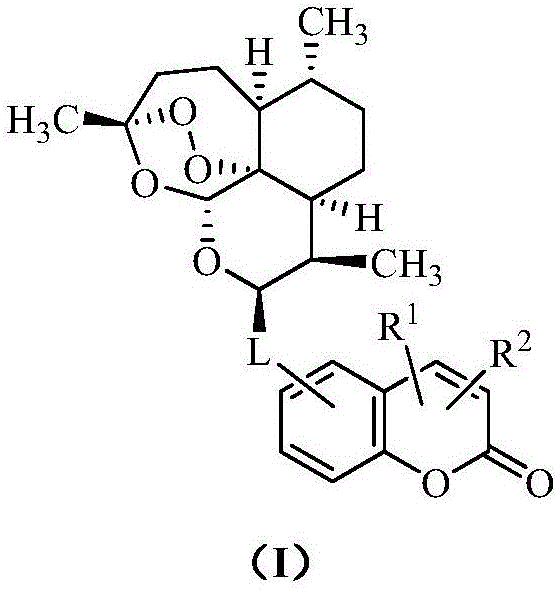
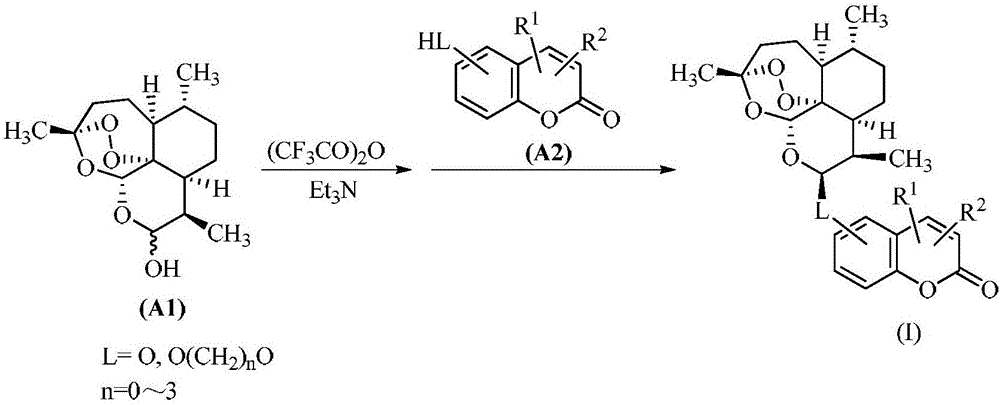
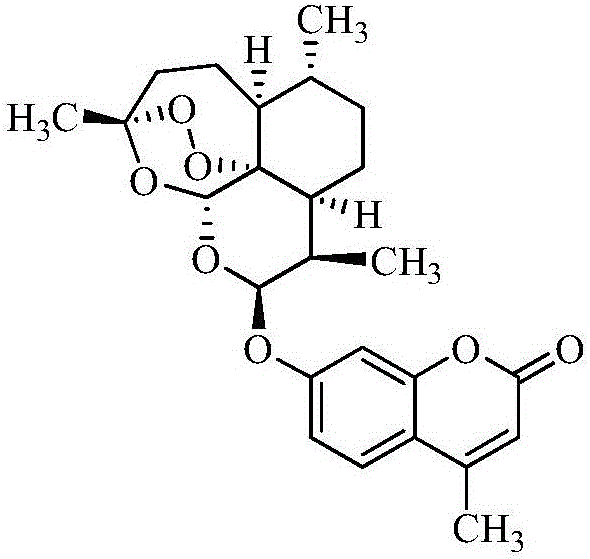
Artemisinin-coumarin heterozygous molecule, method for preparing same and application of artemisinin-coumarin heterozygous molecule
A technology of dihydroartemisinin and halogen, applied in the directions of active ingredients of heterocyclic compounds, drug combinations, organic chemistry, etc., can solve the problems of poor water solubility, low bioavailability, and limited clinical application.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0058] Experimental example 1: Preparation of 7-{[(10S)-dihydroartemisinin-10-oxyl]}-4-methyl-2H-benzothiopyran-2-one (TY-1)
[0059]
[0060] Add 2.8g (0.01mol) of dihydroartemisinin and 1.01g (0.01mol) of triethylamine into a 100mL reaction flask, add 30mL of dry dichloromethane, stir to dissolve, cool to -5°C, and drop Add 1.4mL (0.01mol) trifluoroacetic anhydride, after dropping, continue to react at 0°C for 8 hours to obtain a dichloromethane solution of 10-trifluoroacetoxydihydroartemisinin, which can be used directly for Next reaction. 1.8 g (0.01 mol) of 7-hydroxy-4-methylcoumarin was added to the solution prepared above, and the stirring reaction was continued at room temperature for 24 h after the addition was completed (monitored by TLC at the end of the reaction). Add 30 mL of dichloromethane to the reaction solution, separate the organic layer, and wash with saturated sodium bicarbonate solution 5 times (5 × 20 mL), dry the organic phase with anhydrous sodium ...
experiment example 2
[0061] Experimental example 2: Preparation of 7-{[(10S)-dihydroartemisinin-10-oxyl]}-4-trifluoromethyl-2H-benzothiopyran-2-one (TY-2)
[0062]
[0063] Add 2.8g (0.01mol) of dihydroartemisinin and 1.01g (0.01mol) of triethylamine into a 100mL reaction flask, add 30mL of dry dichloromethane, stir to dissolve, cool to -5°C, and drop Add 1.4mL (0.01mol) of trifluoroacetic anhydride, after dropping, continue to stir and react at 0°C for 8 hours to obtain a dichloromethane solution of 10-trifluoroacetoxydihydroartemisinin, without further treatment, directly use react in the next step. 2.3 g (0.01 mol) of 7-hydroxy-4-trifluoromethylcoumarin was added to the solution prepared above, and the stirring reaction was continued at room temperature for 14 hours after the addition was completed (the end point of the reaction was monitored by TLC). Add 30 mL of dichloromethane to the reaction solution, separate the organic layer, and wash with saturated sodium bicarbonate solution 5 time...
experiment example 3
[0064] Experimental example 3: Preparation of 7-{[(10S)-dihydroartemisinin-10-oxyl]}-4-nitro-2H-benzothiopyran-2-one (TY-3)
[0065]
[0066] Add 2.8g (0.01mol) of dihydroartemisinin and 1.01g (0.01mol) of triethylamine into a 100mL reaction flask, add 30mL of dry dichloromethane, stir to dissolve, cool to -5°C, and drop Add 1.4mL (0.01mol) of trifluoroacetic anhydride, after dropping, continue to stir and react at 0°C for 8 hours to obtain a dichloromethane solution of 10-trifluoroacetoxydihydroartemisinin, without further treatment, directly use react in the next step. 2.1 g (0.01 mol) of 7-hydroxy-4-nitrocoumarin was added to the solution prepared above, and the stirring reaction was continued at room temperature for 5 hours after the addition was completed (the end of the reaction was monitored by TLC). Add 30 mL of dichloromethane to the reaction solution, separate the organic layer, and wash with saturated sodium bicarbonate solution 5 times (5 × 20 mL), dry the orga...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com