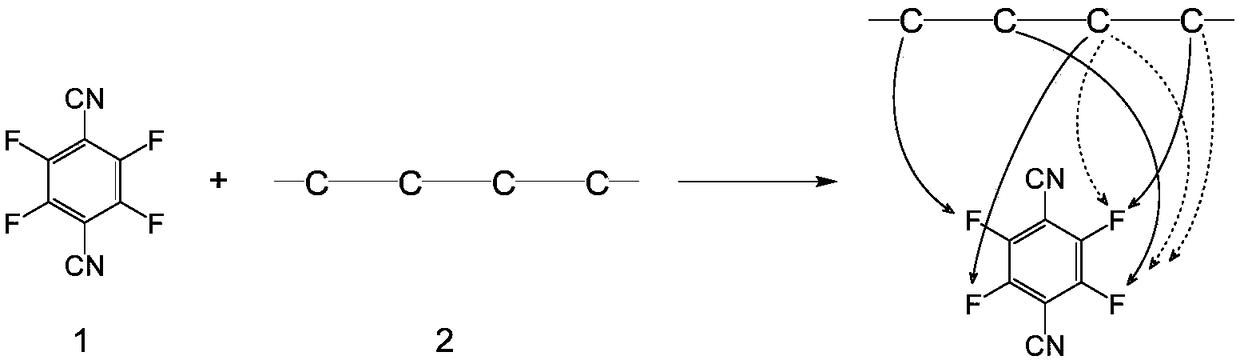
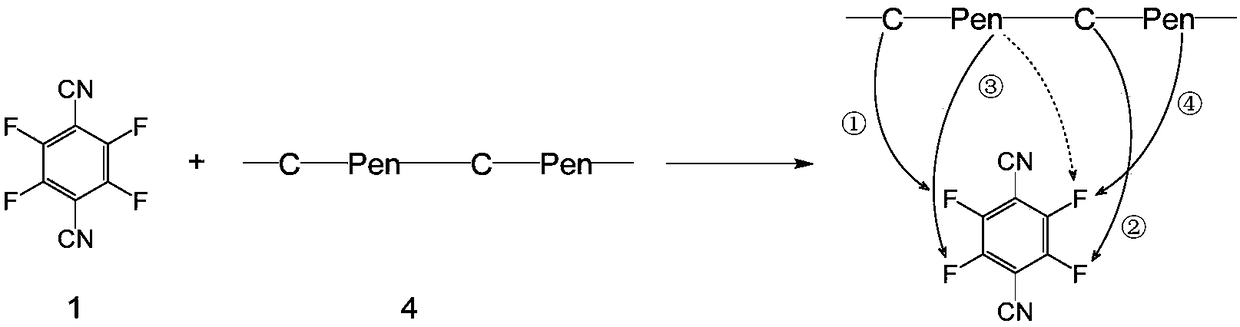
A method for constructing stable three-membered cyclic peptide based on tetrafluorophthalonitrile
A technology of tetrafluorophthalonitrile and three-membered rings, which is applied in the field of polycyclic peptides, can solve the problems of complex products and difficult regulation, and achieve the effect of single product, high yield and precise structure regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
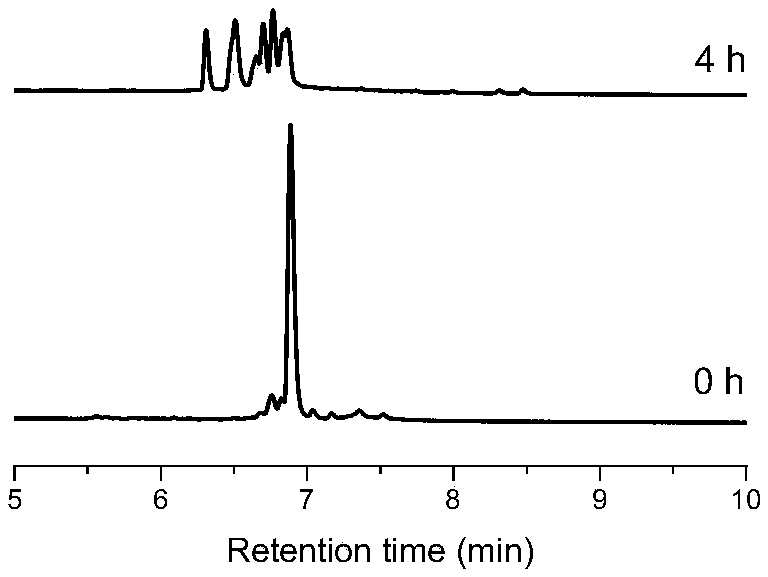
[0031] Dissolve WGCKCGGKGGCGKGGGCGW (amidation and amination at both ends) in water, react 50 μM polypeptide with 200 μM compound 1 in 100 mM pH=7.4 phosphate buffered aqueous solution for 4 hours at room temperature, and obtain 6 tetrasubstituted products with a total yield of 70 %, its chromatogram is as image 3 . The corresponding three-membered cyclic peptide was obtained after purification, separation and freeze-drying by high performance liquid chromatography, and the resulting product was characterized by mass spectrometry m / z: 960.0 (M 2+ ),640.3(M 3+ ).
Embodiment 2
[0033] Dissolve WGCGGKGGPenGKGGGCGKGGGPenGW (amidation and amination at both ends respectively) in water, react 50 μM polypeptide with 200 μM compound 1 in 100 mM pH=7.4 phosphate buffered aqueous solution for 8 hours at room temperature, and obtain two tetrasubstituted products, the chromatograms are as follows Figure 4 (After 8h, two tetrasubstituted products were mainly obtained, the yield was 85%). The corresponding two three-membered cyclic peptides were obtained after purification, separation and lyophilization by high-performance liquid chromatography. The resulting product is characterized by mass spectrometry as m / z: 1102.8 (M 2+ ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com