Azilsartan kaMedoxoMil solid dispersoid preparation and preparation method thereof
A technology of azilsartan medoxomil and solid dispersion, which is applied in the field of azilsartan medoxomil potassium salt solid dispersion preparation and its preparation, and can solve the problems that Edarbi cannot be replaced in terms of effectiveness, safety and tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
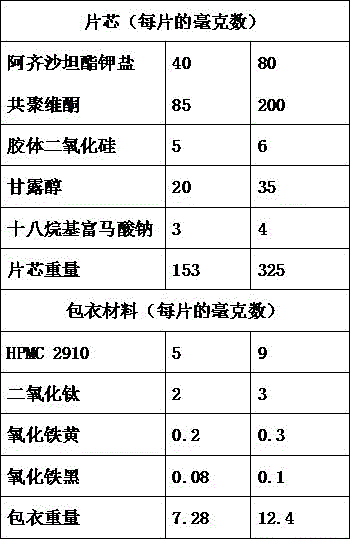
[0040] Embodiment 1: Azilsartan medoxomil potassium salt solid dispersion tablet
[0041] prescription:
[0042] components
parts by weight
Azilsartan medoxomil potassium salt
40
Povidone K30
85
Microcrystalline Cellulose 102
50
20
8
silica
2
Sheet weight
215
[0043] Preparation process: solvent evaporation method
[0044] Dissolve the prescribed amount of azilsartan medoxomil potassium salt and povidone K30 in a solvent of acetone:methanol (1:3), volatilize under reduced pressure in a water bath at 60°C, vacuum degree 0.07-0.08MPa, reduce The organic solvent was recovered under pressure, and after it became viscous, it was dried under reduced pressure for 1 hour, transferred to a vacuum drying oven, dried at 40°C for 48 hours, and then crushed through an 80-mesh sieve to obtain a solid dispersion.
[0045] The prepared solid dispersion was ad...
Embodiment 2
[0046] Embodiment 2: Azilsartan medoxomil potassium salt solid dispersion tablet
[0047] prescription:
[0048] components
parts by weight
Azilsartan medoxomil potassium salt
80
Povidone K30
150
Microcrystalline Cellulose 102
90
20
7
talcum powder
3
Sheet weight
350
[0049] Preparation process: solvent evaporation method
[0050] Take the prescribed amount of azilsartan medoxomil potassium salt and povidone K30, dissolve in methanol: dichloromethane (4:1) solvent, put in water bath at 60°C, vacuum degree 0.07-0.08MPa, and recover organic After the solvent becomes viscous, continue vacuum drying under reduced pressure for 1 hour, transfer to a vacuum drying oven, dry at 40°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0051] The prepared solid dispersion is added into the prescribed amount of mi...
Embodiment 3
[0052] Embodiment 3: Azilsartan medoxomil potassium salt solid dispersion tablet
[0053] prescription:
[0054] components
parts by weight
Azilsartan medoxomil potassium salt
20
Povidone K30
60
Microcrystalline Cellulose 102
30
10
7
silica
3
Sheet weight
130
[0055] Preparation process: solvent evaporation method
[0056] Dissolve the prescribed amount of azilsartan medoxomil potassium salt and povidone K30 in a solvent of acetone:dichloromethane (3:1), in a water bath at 55°C, with a vacuum of 0.07-0.08MPa, and recover organic matter under reduced pressure. After the solvent becomes viscous, continue vacuum drying under reduced pressure for 3 hours, transfer to a vacuum drying oven, dry at 60°C for 48 hours, and pass through an 80-mesh sieve to pulverize to obtain a solid dispersion.
[0057] The prepared solid dispersion is added to the presc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com