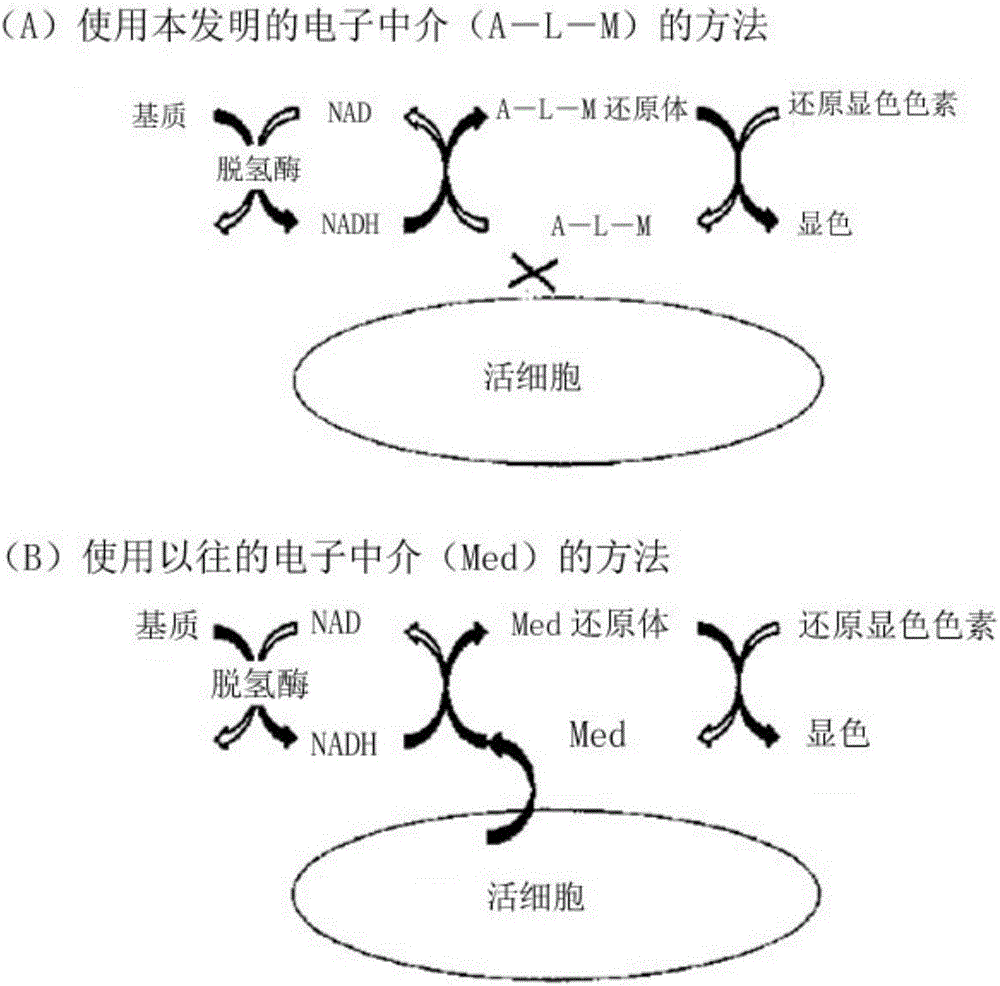
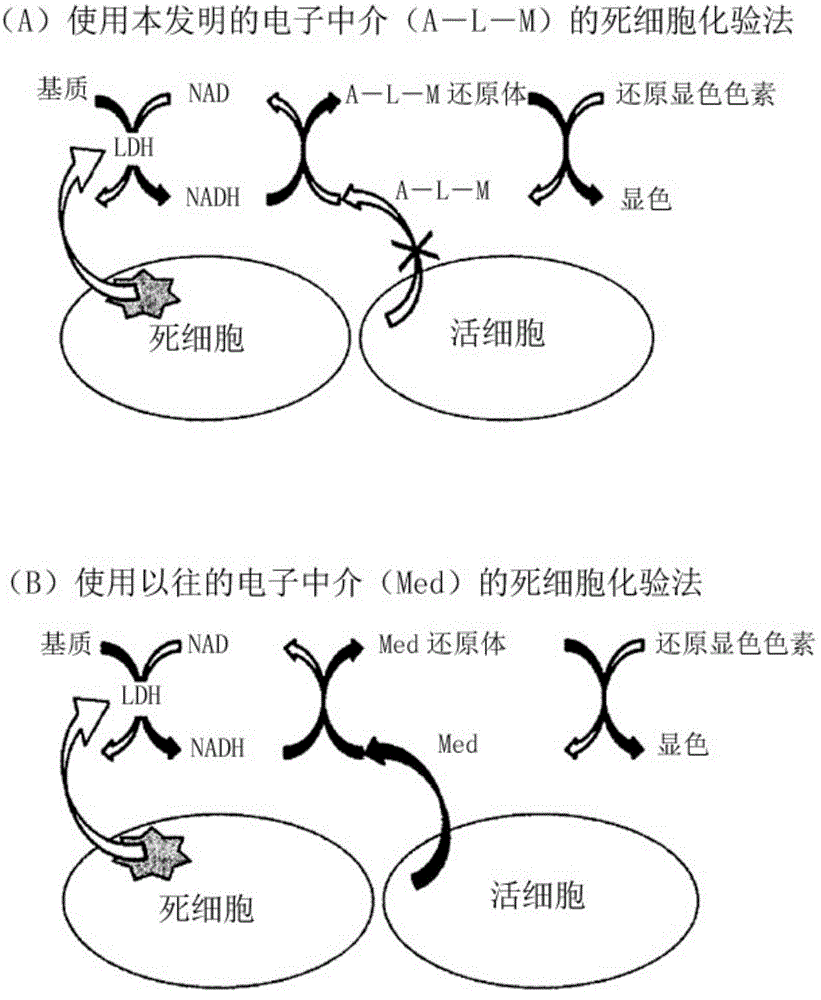
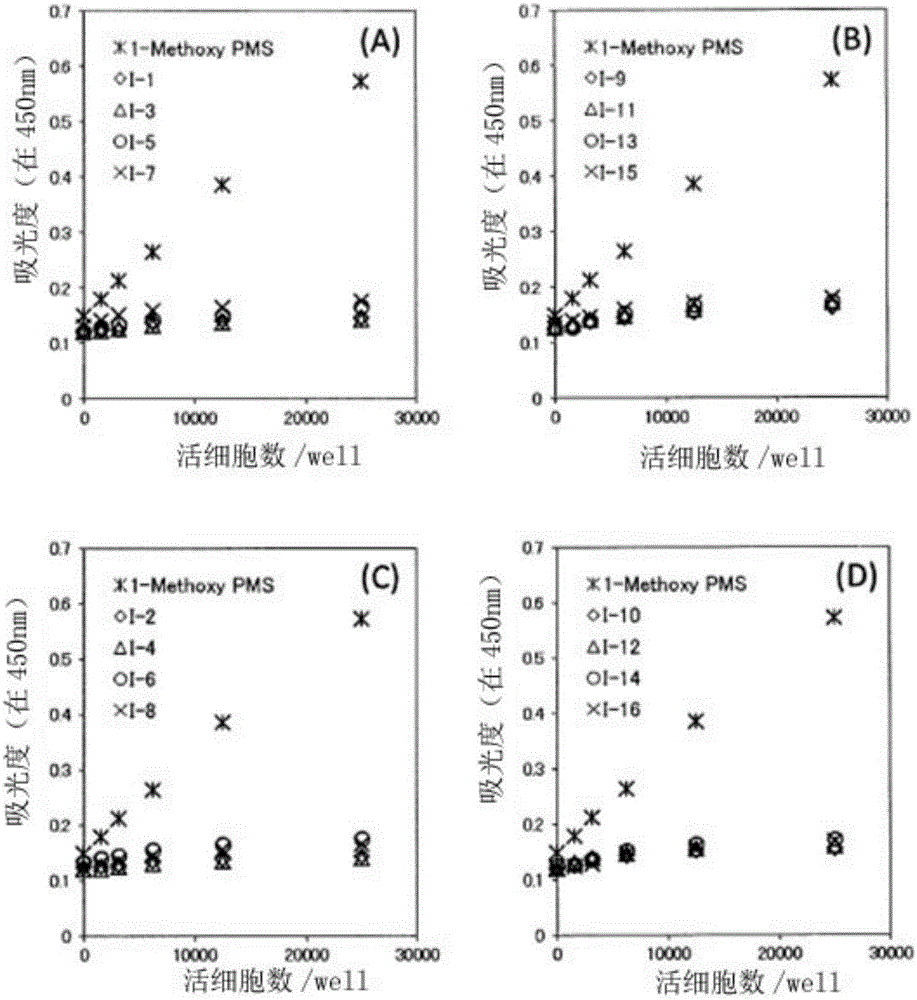
Measurement method for dehydrogenase and substrate concentration thereof, electron mediator, and measurement reagent for dehydrogenase including said electron mediator and for substrate thereof
一种电子中介、测定方法的技术,应用在微生物的测定/检验、生物测试、生物化学设备和方法等方向,能够解决不容易使用、试剂溶液安定性差等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: Synthesis of Ⅰ-1
[0065] [chemical formula 8]
[0066]
[0067] The 5-Methyl-1-carboxybutyl phenazine derivative (1) was synthesized according to the method already reported (International Publication No. 2009 / 118157). 40mg (50μmol) of N 6 ―2AE―NAD (Tongjin Institute of Chemistry) was dissolved in 1ml of 100mmol / L phosphate buffer solution (pH 7.4), and 40mg (209μmol) of WSC (1-Ethyl-3-(3-dimethylaminopro pyl)carbodiimide hydrochloride) and 85.8 mg (209 μmol) of compound 1 was reacted at room temperature for 20 hours. The reaction mixture was purified by reverse-phase HPLC, and dried under reduced pressure in a desiccator to obtain 12 mg of a red solid of I-1.
Embodiment 2
[0068] Embodiment 2: Synthesis of Ⅰ-4
[0069] [chemical formula 9]
[0070]
[0071] The 5-Ethyl-1-carboxybutyl phenazine derivative (2) was synthesized according to the method already reported (International Publication No. 2009 / 118157). 44.8mg (57μmol) of N 6 ―2A E―NADP (Tongjin Institute of Chemistry) was dissolved in 1ml of 100mmol / L phosphate buffer solution (pH 7.4), and 40mg (209μmol) of WSC (1-Ethyl-3-(3-dimethylaminopro pyl)carbodiimide hydrochloride) and 88.7 mg (209 μmol) of compound 2 was reacted at room temperature for 20 hours. The reaction mixture was purified by reverse-phase HPLC, and dried under reduced pressure in a desiccator to obtain 15 mg of a red solid of I-4.
Embodiment 3
[0072] Embodiment 3: Synthesis of Ⅰ-6
[0073] [chemical formula 10]
[0074]
[0075] The 5-Ethyl-1-carboxybutyl phenazine derivative (2) was synthesized according to the method already reported (International Publication No. 2009 / 118157). Dissolve 40mg (301μmol) of aspartic acid in 1ml of 100mmol / L phosphate buffer solution (pH 7.4), add 40mg (209μmol) of WSC (1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride) and 88.7 mg (209 μmol) of compound 2 was reacted at room temperature for 20 hours. The reaction mixture was purified by reverse-phase HPLC, and dried under reduced pressure in a desiccator to obtain 45 mg of a red solid of I-6.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com