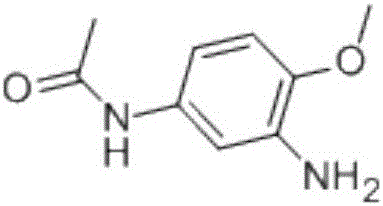
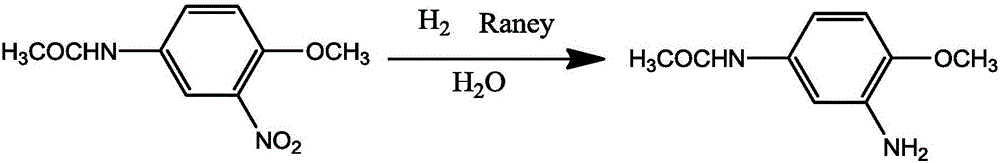
Method for preparing 2-amino-4-acetaminoanisole
A technology of acetamidoanisole and amino group, applied in the chemical industry, can solve problems such as high risk factor, potential safety hazard, increase product cost, etc., and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A method for preparing 2-amino-4-acetamidoanisole by hydrogenation reduction of 2-nitro-4-acetamidoanisole in water phase, in a closed reaction kettle, adding 2-nitro-4-acetamido Anisole 50g, water 500mL, Raney nickel 5g, stir and add acid-binding agent liquid caustic soda (3%) to adjust the pH value to 7.5, heat up to 95 ° C, when the system pressure drops to 2.0MPa, open the hydrogen valve, keep The hydrogen pressure is 3.0MPa. After reacting for 3-4 hours under this pressure and temperature condition, close the hydrogen valve and observe the pressure change in the kettle. If the pressure drops, continue to feed hydrogen until there is no pressure change. After the reaction, stop heating, filter while it is hot, separate and recover the catalyst, filter the filtrate to obtain 2-amino-4-acetamidoanisole after cooling and precipitation, and obtain 41.7g light brown granular product, 2-amino-4-acetamidobenzene The purity of methyl ether is 99.38%, the content is 96.67%, ...
Embodiment 2
[0035] A method for preparing 2-amino-4-acetamidoanisole by hydrogenation reduction of 2-nitro-4-acetamidoanisole in water phase, in a closed reaction kettle, adding 2-nitro-4-acetamido Anisole 50g, water 600mL, Raney nickel 5g, stir and add acid-binding agent liquid caustic soda (3%) to adjust the pH value to 7.5, heat up to 95 ° C, when the system pressure drops to 2.0MPa, open the hydrogen valve, keep The hydrogen pressure is 3.0MPa. After reacting for 3-4 hours under this pressure and temperature condition, close the hydrogen valve and observe the pressure change in the kettle. If the pressure drops, continue to feed hydrogen until there is no pressure change. After the reaction, stop heating, filter while it is hot, separate and recover the catalyst, filter the filtrate to obtain 2-amino-4-acetamidoanisole after cooling and precipitation, and obtain 41.3g of light brown granular product, 2-amino-4-acetamidobenzene The purity of methyl ether is 99.24%, the content is 96.73...
Embodiment 3
[0037] A method for preparing 2-amino-4-acetamidoanisole by hydrogenation reduction of 2-nitro-4-acetamidoanisole in water phase, in a closed reaction kettle, adding 2-nitro-4-acetamido Anisole 50g, water 500mL, Raney nickel 5g, stir and add acid-binding agent soda ash to adjust the pH value to 7.5, heat up to 95°C, when the system pressure drops to 2.0MPa, open the hydrogen valve to keep the hydrogen pressure at 3.0MPa After reacting for 3-4 hours under this pressure and temperature condition, close the hydrogen valve and observe the pressure change in the kettle. If the pressure drops, continue to feed hydrogen until there is no pressure change. After the reaction, stop heating, filter while it is hot, separate and recover the catalyst, filter the filtrate to obtain 2-amino-4-acetamidoanisole after cooling and precipitation, and obtain 41.7g light brown granular product, 2-amino-4-acetamidobenzene The purity of methyl ether is 99.28%, the content is 96.71%, and the product y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com