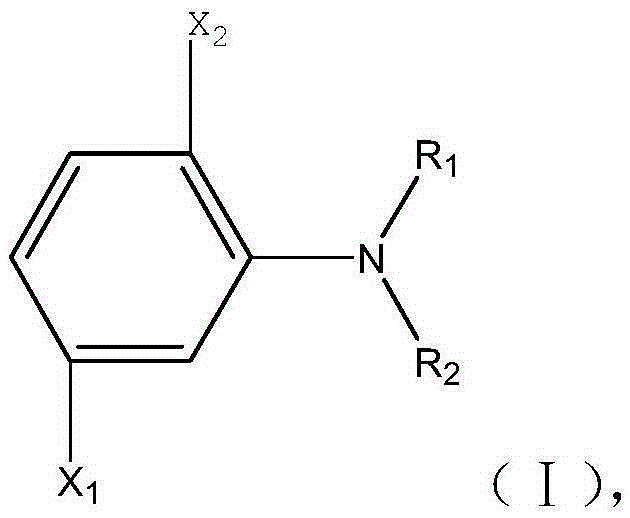
Diazotization preparation method of chloroarylamine
A technology of chlorinated arylamine and diazotization, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid nitrile, etc., to achieve the effect of improving environmental protection performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Diazotization reaction: Add 65% sulfuric acid 33.2g (0.22mol) into the diazonium reaction vessel, cool down to 10°C-15°C, and control the temperature at 10°C-15°C, slowly add 15.5% nitrosyl Sulfuric acid 84.4g (0.103mol), after adding, control the temperature at 20°C to 25°C, slowly add 20.9g (0.1mol) of 99% 2,6-dichloro-4-nitroaniline, and Insulate and react at ℃ for 6-8 hours, and check the end point (take a small amount of diazonium solution and dilute it with ice water, if the solution is clear, it means that the reaction has reached the end point). After the reaction is completed, the prepared 2,6-dichloro-4-nitroaniline diazonium solution is added to 250g of ice water for dilution. After adding the diazo solution, control the temperature below 5°C and keep stirring for 4-6 minutes, then filter to remove impurities. Filtrate to be coupled reaction;
[0018] (2) Coupling reaction: Add 34.16g (0.1mol) of N-cyanoethyl-N-acetoxyethylaniline with a content of 68% i...
Embodiment 2
[0020] (1) Diazotization reaction: Add 40g (0.4mol) of 98% sulfuric acid into the diazonium reaction vessel, cool down to 10°C-15°C, and control the temperature at 10°C-15°C, slowly add 40% nitrosyl sulfuric acid 32.4g (0.102mol), after adding, control the temperature at 20°C to 25°C, slowly add 21.8g (0.1mol) of 95% 2,6-dichloro-4-nitroaniline, and Insulate the reaction for 6-8 hours, and check the end point (take a small amount of diazonium solution and dilute it with ice water, and the clear solution indicates that the reaction has reached the end point). After the reaction is completed, the prepared 2,6-dichloro-4-nitroaniline diazonium solution is added to 300g of ice water for dilution. After adding the diazo solution, control the temperature below 0°C and keep stirring for 4-6 minutes, filter to remove impurities, and filter Liquid to be coupled reaction;
[0021] (2) Coupling reaction: Add 27.3 g (0.1 mol) of 73% N,N-dicyanoethylaniline to 400 g of water, add the filt...
Embodiment 3~16
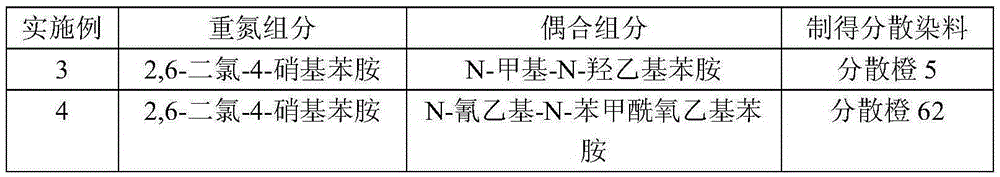
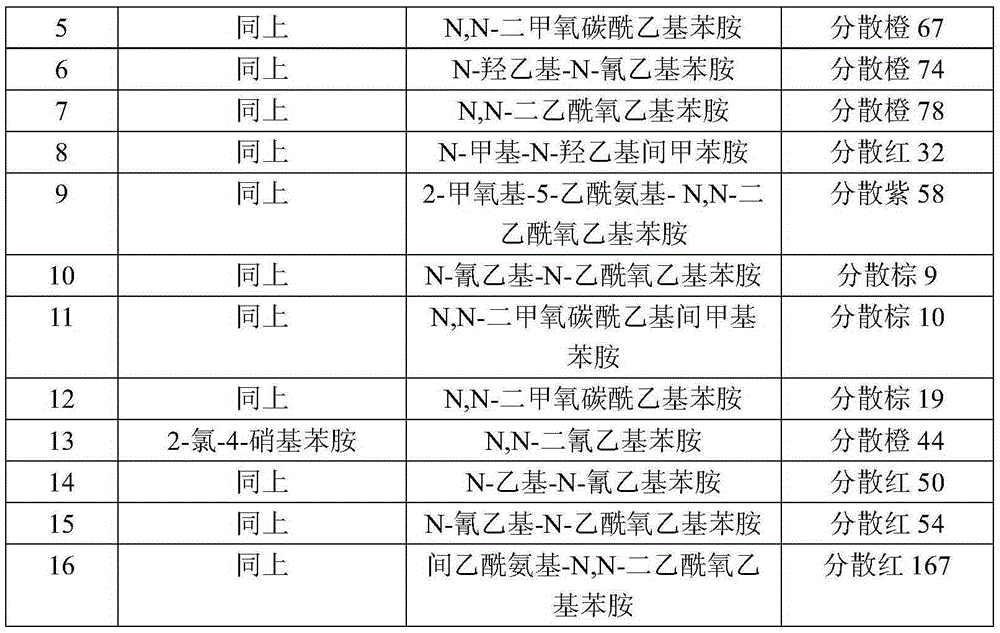
[0023] According to the preparation method of Example 1, the difference is that the diazo component and coupling component A in the following Table 1 are used in equimolar amounts to prepare conventional disperse dyes.
[0024] Table 1
[0025]
[0026]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com