Preparation method of ferrous sulfate skeleton type sustained-release dropping pills
A technology of ferrous sulfate and sustained-release dropping pills, which is applied in the fields of pill delivery, pharmaceutical formulations, extracellular fluid diseases, etc. and other problems, to achieve the effect of improving patient compliance, good drug release effect, and simple calculation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
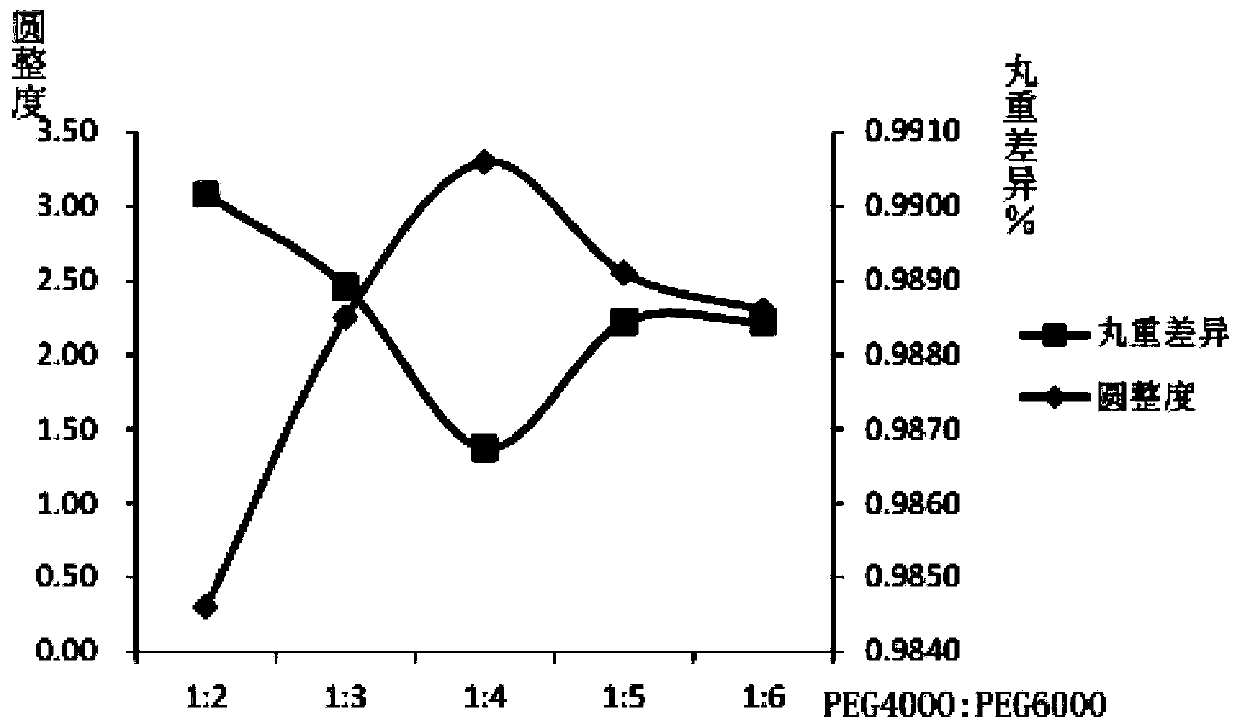
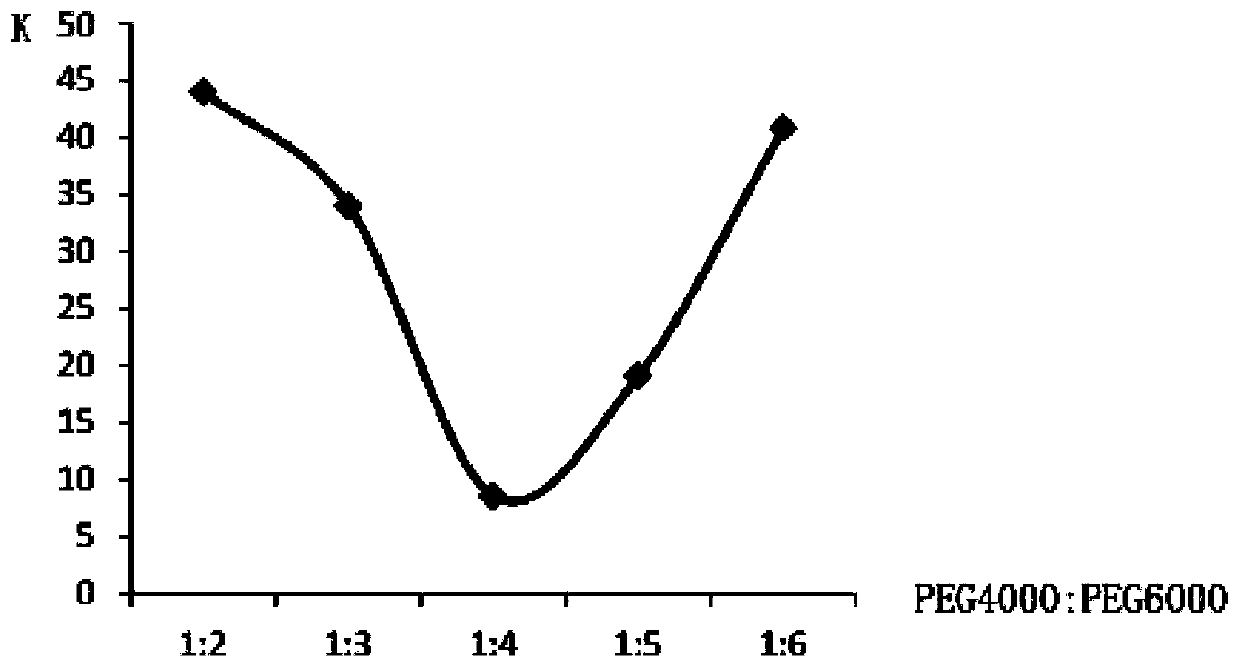
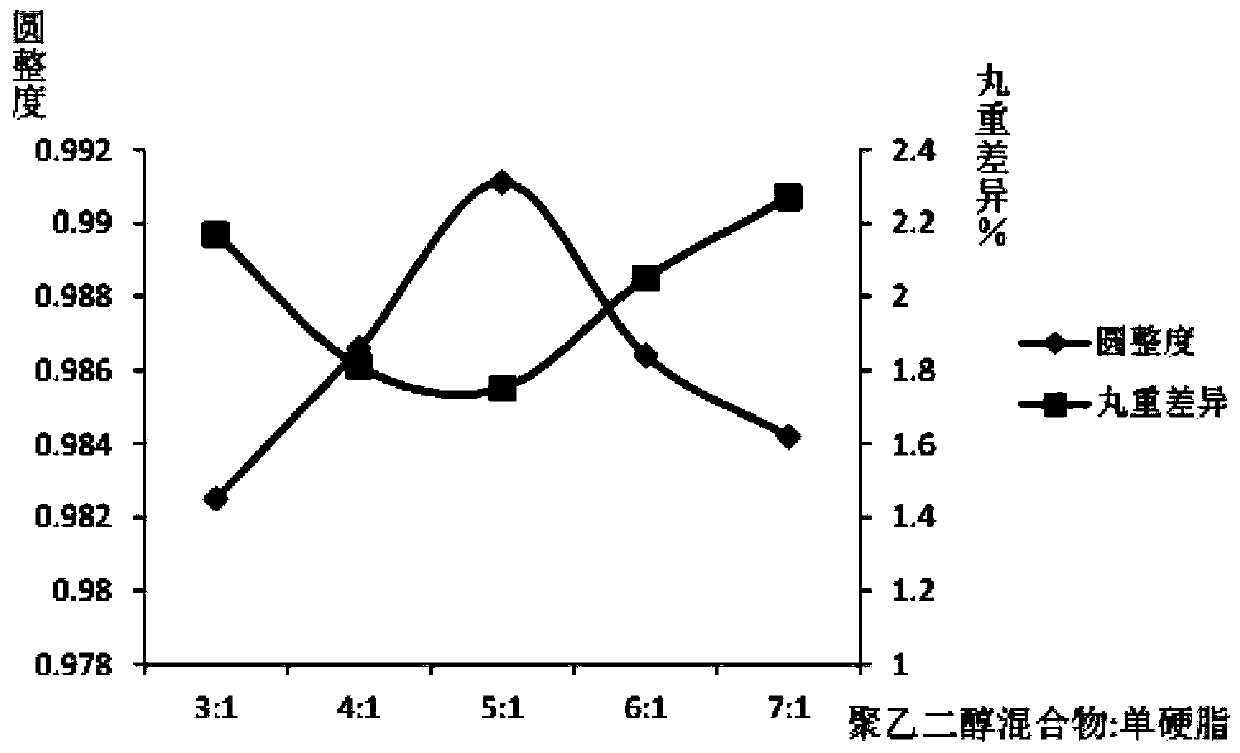
[0074] A preparation method of a ferrous sulfate skeleton type sustained-release dropping pill, which comprises the following steps: weighing a matrix and drug ferrous sulfate in a weight ratio of 2:1, heating and melting the matrix in a water bath, and then adding ferrous sulfate to the matrix , stirring and dissolving to obtain the liquid medicine, the temperature of the liquid medicine is kept at 70°C, and then the liquid medicine is dripped into the condensate at a drop distance of 2cm and a drop speed of 10 drops / min, and the temperature of the lower half of the condensate is 0°C , into a pellet, take it out after standing, wipe off the condensate, and dry it to get the product; the matrix is a mixture of polyethylene glycol and glyceryl monostearate, and the weight ratio of the mixture of polyethylene glycol and glyceryl monostearate is 3:1, the polyethylene glycol mixture consists of PEG4000 and PEG6000, the weight ratio of PEG6000 and PEG4000 is 2:1.
Embodiment 2
[0076] A preparation method of ferrous sulfate skeleton type sustained-release dripping pills, which comprises the following steps: weighing a matrix and drug ferrous sulfate in a weight ratio of 4:1, heating and melting the matrix in a water bath, and then adding ferrous sulfate to the matrix , stirring and dissolving to obtain the liquid medicine. The temperature of the liquid medicine is kept at 85°C, and then the liquid medicine is dripped into the condensate at a drop distance of 6cm and a drop speed of 30 drops / min. The temperature of the lower half of the condensate is 5°C. Form a pellet, take it out after standing, wipe off the condensate, and dry it to get the product; the matrix is a mixture of polyethylene glycol and glyceryl monostearate, and the weight ratio of the mixture of polyethylene glycol and glyceryl monostearate is 5 :1, the polyethylene glycol mixture is composed of PEG4000 and PEG6000, and the weight ratio of PEG6000 and PEG4000 is 4:1.
Embodiment 3
[0078] A preparation method of ferrous sulfate skeleton type sustained-release dripping pills, which comprises the following steps: weighing base and drug ferrous sulfate in a weight ratio of 6:1, heating and melting the base in a water bath, and then adding ferrous sulfate into the base , stirring and dissolving to obtain the liquid medicine. The temperature of the liquid medicine is kept at 90°C, and then the liquid is drawn into the condensate at a drop distance of 10cm and a drop speed of 50 drops / min. The temperature of the lower half of the condensate is 20°C. Form a pellet, take it out after standing, wipe off the condensate, and dry it to get the product; the matrix is a mixture of polyethylene glycol and glyceryl monostearate, and the weight ratio of the mixture of polyethylene glycol and glyceryl monostearate is 7 :1, the polyethylene glycol mixture is composed of PEG4000 and PEG6000, and the weight ratio of PEG6000 and PEG4000 is 6:1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com