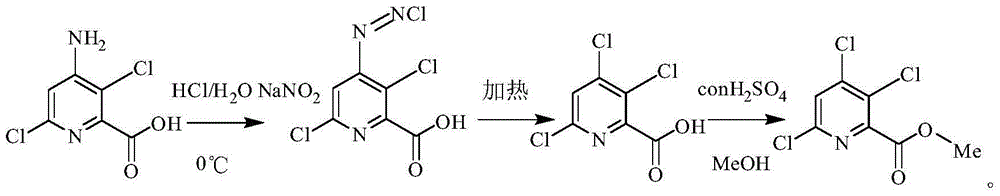
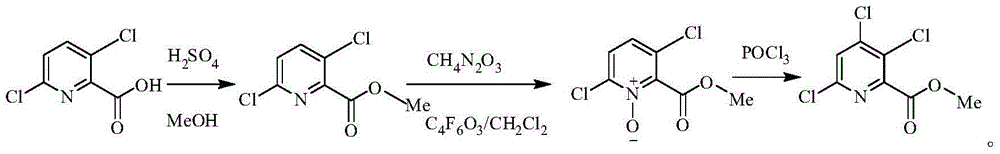Preparing method of 3,4,6-trichloropyridine-2-formic acid and ester thereof
A technology of triclopyridine and picolinic acid, which is applied in the direction of organic chemistry, can solve the problems of low yield, long synthesis line, high production cost, etc., and achieve the effects of high yield, low production cost and poor safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Preparation of 3,4,6-trichloropyridine-2-carboxylic acid
[0025] Add 240ml of water and 90ml of concentrated hydrochloric acid to a 1L reaction flask, and add 108.68g (0.525mol) of 3,6-dichloro-4amino-2-pyridinecarboxylic acid into the reaction flask under stirring, then slowly Add dropwise sodium nitrite solution (this sodium nitrite solution contains 51g (0.6mol) sodium nitrite and 90ml water), after dropwise addition, keep stirring until 3,6-dichloro-4 amino-2-pyridinecarboxylic acid disappears, A reaction solution was obtained. Slowly heat the reaction solution to 60°C to 100°C, preferably 75°C to 80°C, keep it warm for 3 hours, then cool down to room temperature, then extract three times with ethyl acetate, the amount of ethyl acetate each time is 120mL, and combine the organic phases. The organic phase was washed with water, concentrated, and then recrystallized to obtain 3,4,6-trichloropyridine-2-carboxylic acid with a content of 98%.
[0026] Under ...
Embodiment 2
[0029] Example 2 Preparation of 3,4,6-trichloropyridine-2-carboxylate
[0030] Add 10g (0.0444mol) of 3,4,6-trichloropyridine-2-carboxylic acid to a 100mL reaction flask, then add 20g of methanol and 1g of concentrated sulfuric acid, and heat up to reflux for 16 hours. After the reaction is complete, distill out the methanol under reduced pressure, and then Then collect the fraction at 155-160°C under the condition of -0.098MPa. Add methanol three times the mass of the fraction to the fraction, heat to reflux, cool to 0°C, and filter under reduced pressure to obtain the mother liquor. After the mother liquor was spin-dried, it was beaten with methanol, cooled and filtered with suction to obtain 9.5 g of methyl 3,4,6-trichloropyridine-2-carboxylate as a white solid.
[0031] It is determined that the mp (melting point, referred to as: melting point) of the prepared white solid is 60-63°C, 1 HNMR (400MHz, CDCl3) δ 7.58 (s, 1H), 4.01 (s, 3H) ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com