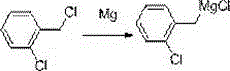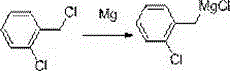Synthetic method of o-chlorobenzylmagnesium chloride
A technology of o-chlorobenzylmagnesium chloride and its synthesis method, which is applied in the direction of magnesium organic compounds, etc., and can solve the problems of poor control of reaction temperature, difficulty in realizing industrial application, and low boiling point of solvents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1: In a 2000 mL four-neck flask, add 45.3 g of newly produced magnesium chips, 0.1 g of iodine and 100 g of cyclopentyl methyl ether, and replace with nitrogen three times while stirring. While stirring, under the protection of nitrogen, add 10 g o-chlorobenzyl chloride at one time, the reaction is initiated within about 5-15 minutes, and the temperature begins to rise to 40°C. Add 500 g of cyclopentyl methyl ether within 10 minutes, and when the temperature reaches 25°C, turn on the ice-water bath, and start to drop a solution of o-chlorobenzyl chloride (190 g of o-chlorobenzyl chloride dissolved in 500 g of cyclopentyl methyl ether) base ether), and control the temperature between 20-30°C. After about 3 hours of dripping, after 30 minutes of dripping, the sample was quenched and detected by GC. The yield of the obtained o-chlorobenzyl magnesium chloride was 97%, and the purity was 95%.
Embodiment 2
[0023] Example 2: In a 2000 mL four-neck flask, add 45.3 g of newly produced magnesium chips, 0.1 g of iodine and 100 g of cyclopentyl methyl ether, and replace with nitrogen three times while stirring. While stirring, under the protection of nitrogen, add 10 g o-chlorobenzyl chloride at one time, the reaction is initiated within about 5-15 minutes, and the temperature begins to rise to 40°C. Add 500 g of xylene within 10 minutes, and when the temperature reaches 25°C, turn on the ice-water bath, and start to add a solution of o-chlorobenzyl chloride (190 g of o-chlorobenzyl chloride dissolved in 500 g of toluene), and control the temperature at 20- between 30°C. After about 3 hours of dripping, after 30 minutes of dripping, the sample was quenched and detected by GC. The yield of the obtained o-chlorobenzyl magnesium chloride was 94%, and the purity was 92%.
Embodiment 3
[0024] Example 3: In a 2000 mL four-neck flask, add 45.3 g of newly produced magnesium chips, 0.1 g of iodine and 100 g of 2-methyltetrahydrofuran, and replace with nitrogen three times while stirring. While stirring, under the protection of nitrogen, add 10 g o-chlorobenzyl chloride at one time, the reaction is initiated within about 5-15 minutes, and the temperature begins to rise to 40°C. Add 500 g of 2-methyltetrahydrofuran within 10 minutes. When the temperature reaches 25°C, turn on the ice-water bath and start to add a solution of o-chlorobenzyl chloride (190 g of o-chlorobenzyl chloride dissolved in 500 g of 2-methyltetrahydrofuran ), control the temperature between 20-30°C. After about 3 hours of dripping, after 30 minutes of dripping, the sample was quenched and detected by GC. The yield of the obtained o-chlorobenzyl magnesium chloride was 92%, and the purity was 91%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com