Tin-oxygen-heterocycle-structured dibutyltin 2,4,5-trifluoro-3-methoxy-benzoate complex as well as preparation method and application thereof
A technology of dibutyltin and benzoate, applied in tin organic compounds, compounds of group 4/14 elements of the periodic table, organic chemical methods, etc., can solve the problems of no anticancer activity and high or low anticancer activity, and achieve Good anticancer activity, high anticancer activity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
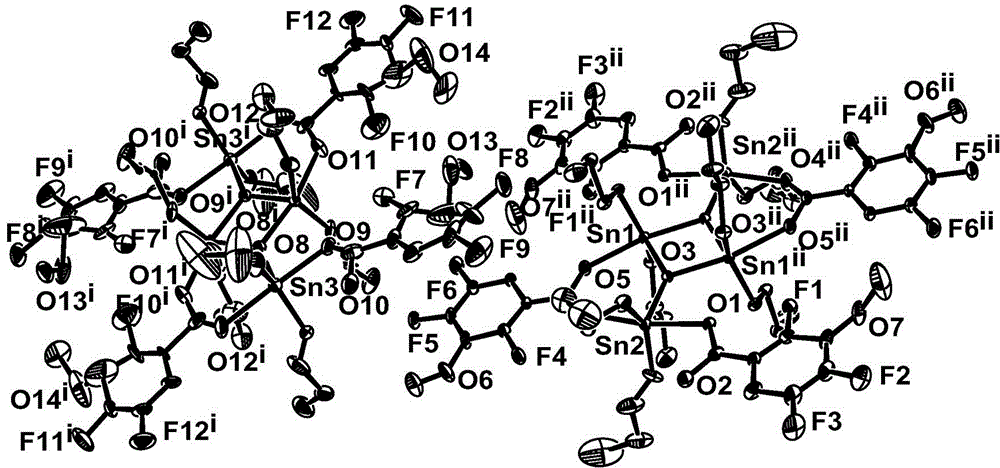
[0033] Preparation of dibutyltin 2,4,5-trifluoro-3-methoxy-benzoate of tin-oxygen heterocyclic structure:
[0034] Add 0.2062g (1mmol) of 2,4,5-trifluoro-3-methoxy-benzoic acid, 0.2493g (1mmol) of dibutyltin oxide, and 20mL of solvent ethanol in sequence in a 100ml round-bottomed flask. React at 50~65°C for 8 hours; cool, filter, and control solvent volatilization and crystallization at 20~35°C to obtain light yellow transparent crystals, which are dibutyltin 2,4,5-trifluoro-3-methanol Oxy-benzoate complexes. Yield: 78%, melting point: 124-125°C.
[0035] Elemental analysis (C 128 h 176 o 28 f 24 sn 8 ): theoretical value: C, 43.08; H, 4.97. Found: C, 43.11; H, 4.95.
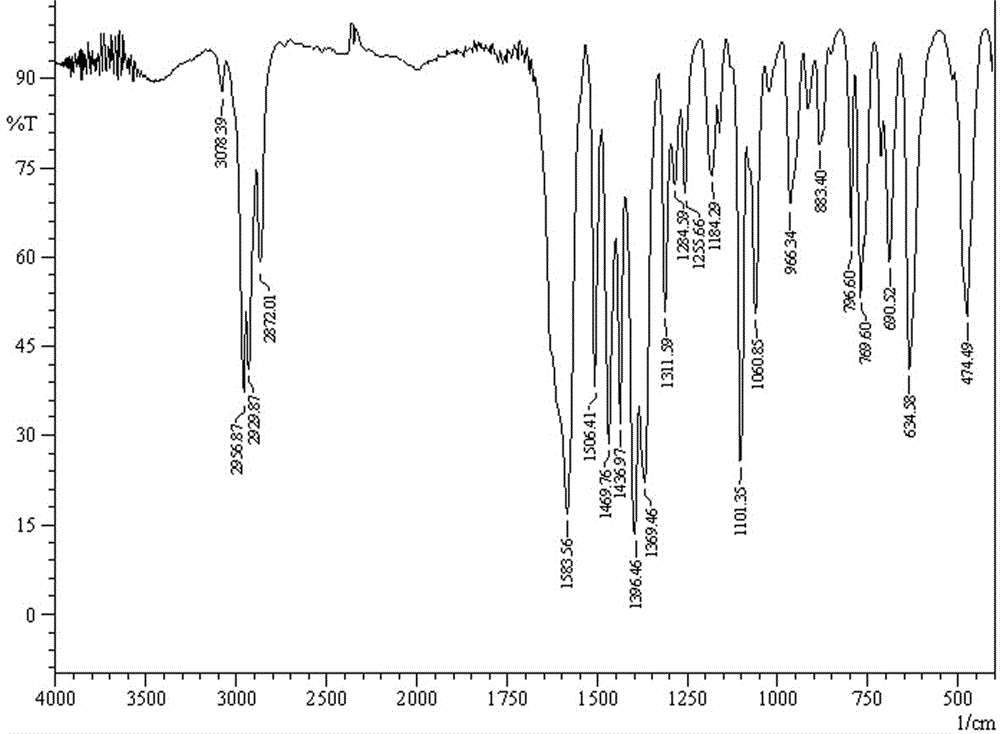
[0036] IR(KBr, cm -1 ): 3078, 2957, 2930, 2872 v(C-H), 1583 v as (COO - ), 1396v as (COO - ), 635 v(Sn-C), 474 v(Sn-O).
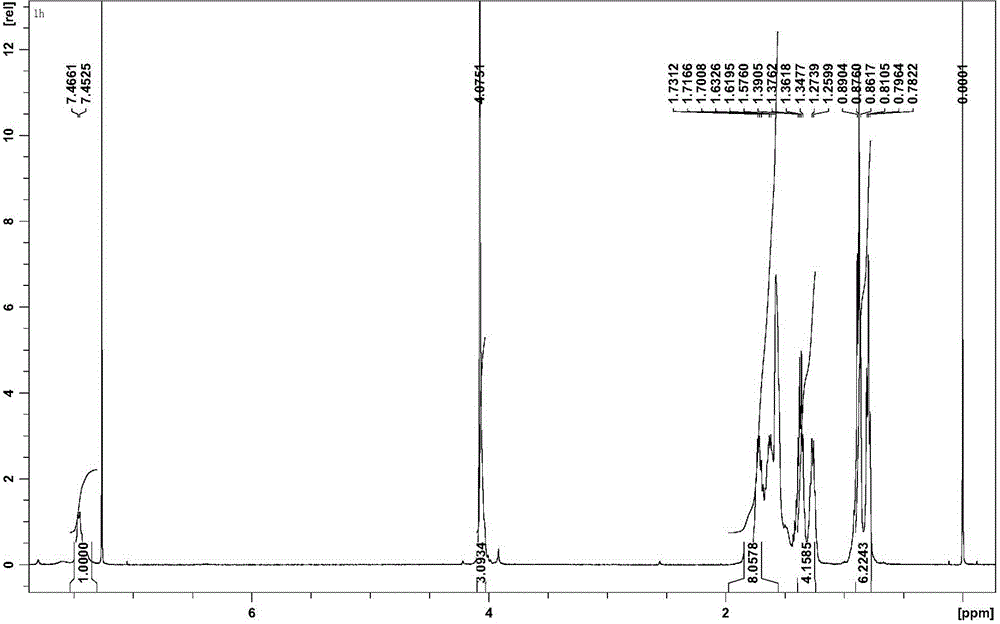
[0037] 1H NMR (CDCl 3 , 500 MHz), δ(ppm): 7.45(d, 8H, Ar-H), 4.08(s, 24H, -O-CH 3 ), 0.78-1.73 (m, 144H, Bu-H).
[0038] 119 Sn NMR (CDCl 3 , 186 MHz), δ(ppm): -212.41, -2...
Embodiment 2
[0041] Preparation of dibutyltin 2,4,5-trifluoro-3-methoxy-benzoate of tin-oxygen heterocyclic structure:
[0042] Add 0.2062g (1mmol) of 2,4,5-trifluoro-3-methoxy-benzoic acid, 0.2490g (1.05mmol) of dibutyltin oxide, and 20mL of solvent ethanol in sequence in a 100ml round bottom flask. React at a temperature of 50~65°C for 8 hours; cool, filter, and control solvent volatilization and crystallization at 20~35°C to obtain light yellow transparent crystals, which are dibutyltin 2,4,5-trifluoro-3- Methoxy-benzoate complex. Yield: 79%, melting point: 124-125°C.
[0043] Elemental analysis (C 128 h 176 o 28 f 24 sn 8 ): theoretical value: C, 43.08; H, 4.97. Found: C, 43.11; H, 4.95.
[0044] IR(KBr, cm -1 ): 3078, 2957, 2930, 2872 v(C-H), 1583 v as (COO - ), 1396v as (COO - ), 635 v(Sn-C), 474 v(Sn-O).
[0045] 1 H NMR (CDCl 3 , 500 MHz), δ(ppm): 7.45(d, 8H, Ar-H), 4.08(s, 24H, -O-CH 3 ), 0.78-1.73 (m, 144H, Bu-H).
[0046] 119 Sn NMR (CDCl 3 , 186 MHz), δ(ppm)...
Embodiment 3
[0049] Preparation of dibutyltin 2,4,5-trifluoro-3-methoxy-benzoate of tin-oxygen heterocyclic structure:
[0050] Add 0.4123g (2mmol) of 2,4,5-trifluoro-3-methoxy-benzoic acid, 0.5220g (2.10mmol) of dibutyltin oxide, and 55mL of solvent ethanol in sequence in a 100ml round bottom flask. React at a temperature of 50~65°C for 18 hours; cool, filter, and control solvent volatilization and crystallization at 20~35°C to obtain light yellow transparent crystals, which are dibutyltin 2,4,5-trifluoro-3- Methoxy-benzoate complex. Yield: 80%, melting point: 124-125°C.
[0051] Elemental analysis (C 128 h 176 o 28 f 24 sn 8 ): theoretical value: C, 43.08; H, 4.97. Found: C, 43.11; H, 4.95.
[0052] IR(KBr, cm -1 ): 3078, 2957, 2930, 2872 v(C-H), 1583 v as (COO - ), 1396v as (COO - ), 635 v(Sn-C), 474 v(Sn-O).
[0053] 1 H NMR (CDCl 3 , 500 MHz), δ(ppm): 7.45(d, 8H, Ar-H), 4.08(s, 24H, -O-CH 3 ), 0.78-1.73 (m, 144H, Bu-H).
[0054] 119 Sn NMR (CDCl 3 , 186 MHz), δ(ppm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com