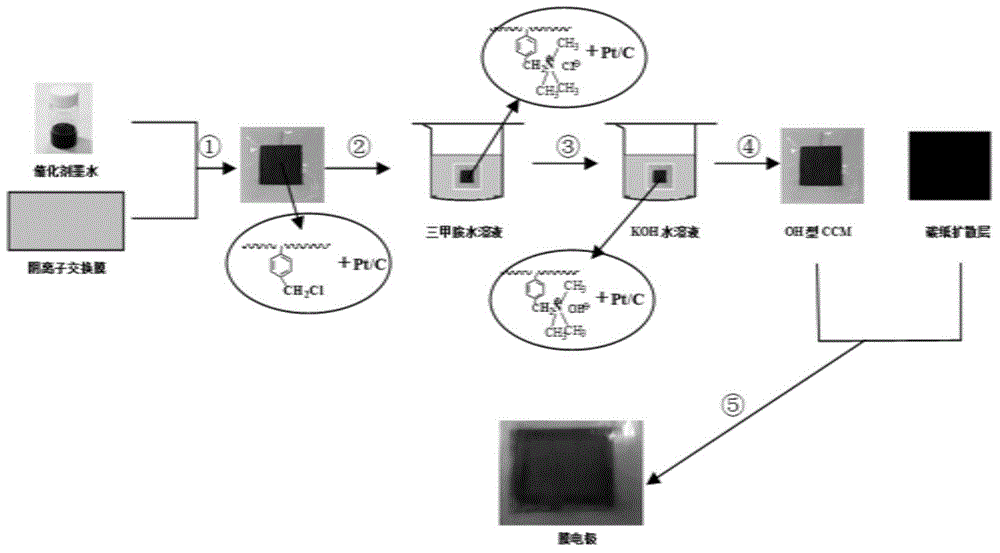
Preparation method for membrane electrode of alkali anion exchange membrane fuel cell
An alkaline anion, fuel cell membrane technology, applied in battery electrodes, circuits, electrical components, etc., to achieve the effect of improving performance and stability, strong adhesion, and promoting conduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Weigh 0.1715 g of 5% chloromethylated resin I-60 solution with an analytical balance, and add 1.6 ml of a mixed solvent of tetrahydrofuran and n-propanol to prepare a polymer resin solution.
[0034] Weigh 34 mg of 70% Pt / C catalyst, add 1.7 ml of n-propanol to ultrasonically disperse for 30 minutes, then add the above-mentioned polymer resin solution to form a catalyst with a mass ratio of 4:1 between electrocatalyst and chloromethylated polymer Ink, ultrasonically dispersed for 60 minutes.
[0035] On a hot stage at 60°C, spray the catalytic layer slurry on the surface of the basic anion exchange membrane, and the area of the sprayed electrode is 24cm 2 , the spraying temperature is room temperature, and the spraying pressure is 0.1MPa. After the solvent is completely evaporated, it is naturally cooled to obtain a catalytic layer film-coated electrode.
[0036] Soak the above-mentioned catalyst-coated membrane electrode in 33% trimethylamine aqueous solution for 2...
Embodiment 2
[0042] The membrane electrode preparation method is as follows:
[0043] Select the self-made alkaline anion exchange membrane 24cm in the group 2 .
[0044] Weigh 0.1715 g of 5% chloromethylated resin I-80 solution by mass fraction, and add 1.6 ml of a mixed solvent of tetrahydrofuran and n-propanol to prepare a polymer resin solution.
[0045] Weigh 34 mg of 70% Pt / C catalyst, add 1.7 ml of n-propanol to ultrasonically disperse for 30 minutes, then add the above-mentioned polymer resin solution to form a catalyst with a mass ratio of 4:1 between electrocatalyst and chloromethylated polymer Ink, ultrasonically dispersed for 60 minutes to make a catalyst slurry.
[0046] On a hot stage at 60°C, spray the catalytic layer slurry on the surface of the basic anion exchange membrane, and spray the electrode area to 24cm 2 , the spraying temperature is room temperature, and the spraying pressure is 0.1MPa.
[0047] After the solvent is completely evaporated, it is naturally cool...
Embodiment 3
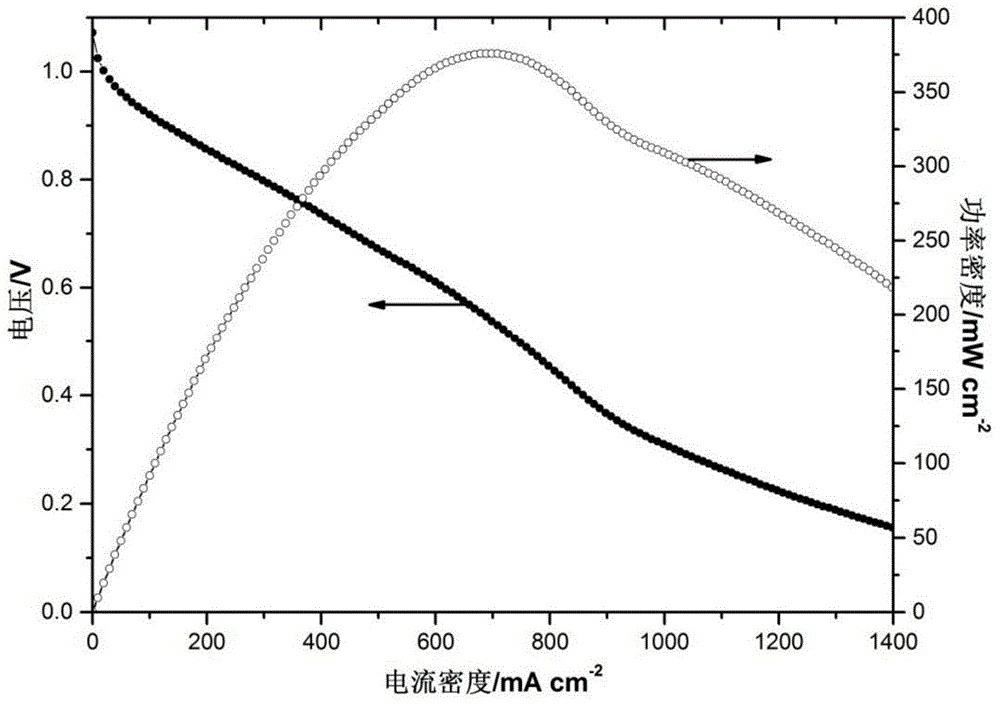
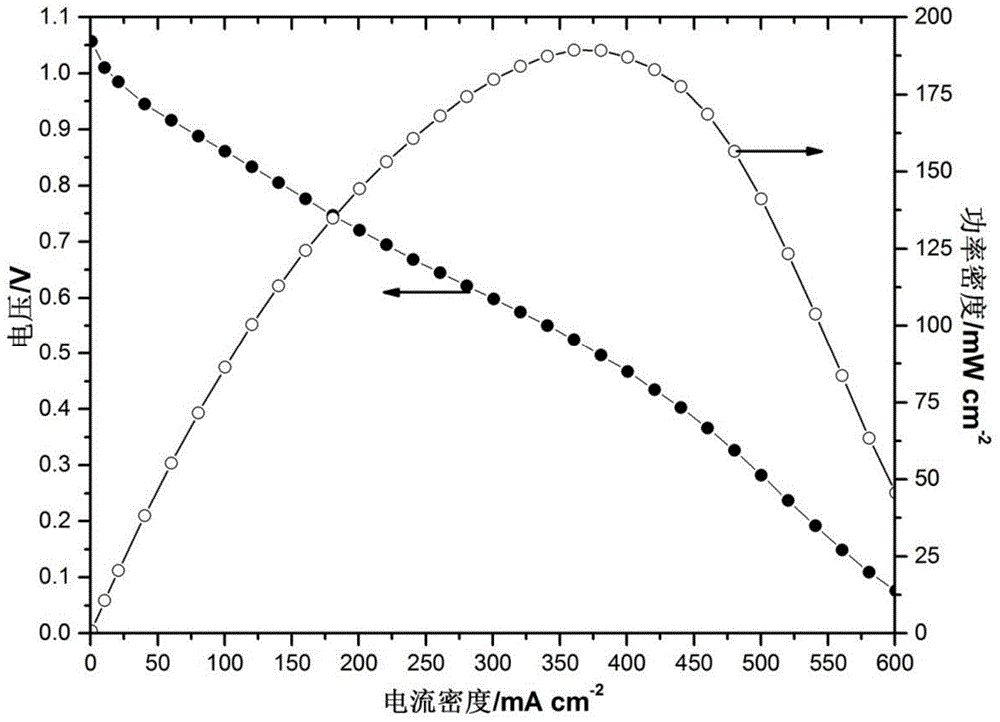
[0056] Different from Example 1, the catalyst on the cathode side uses Fe 3 Co / C, the catalyst loading is 0.4mg / cm 2 . The dispersant is a mixture of tetrahydrofuran and isopropanol, and the mixing ratio is 5:3 (volume ratio); other operations and conditions are the same as in Example 1, and the battery is assembled, and the polarization curve and power density curve of the battery are as follows: Figure 5 As shown, its peak power density is 83mW / cm 2 , compared to the electrode prepared by the traditional amination method (peak power density 63mW / cm 2 ), its peak power density increased by 20mW / cm 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Power density | aaaaa | aaaaa |
| Power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com