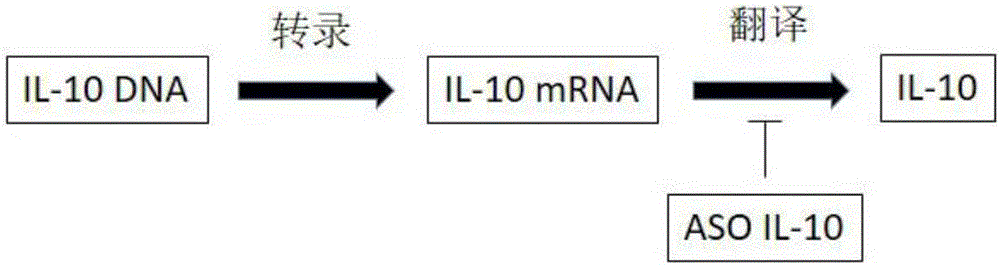
Antisense oligodeoxynucleotide and application thereof
An antisense oligonucleotide and immune adjuvant technology, which can be applied to medical preparations containing active ingredients, biochemical equipment and methods, bacterial antigen components, etc., can solve the problem of no antisense oligonucleotides, etc. To achieve the effect of enhancing the inoculation effect, improving the application effect, and easy to adjust the adjuvant concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: Effect test of immune adjuvant
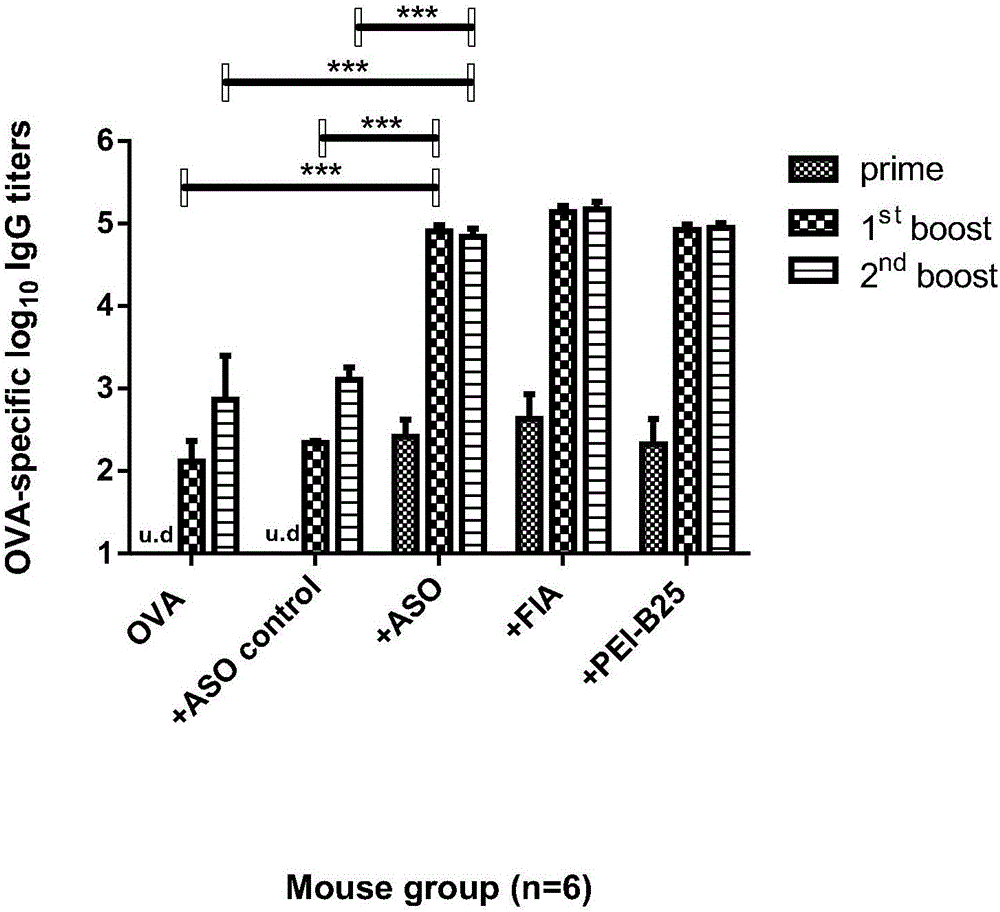
[0061] Experimental method: select inbred strain Balb / c female mice without specific pathogens, aged 6 to 8 weeks, with a weight of 17g±0.5g, 30 were randomly divided into 5 groups, according to body weight, 10 μL of pentobar per gram Bital sodium (10mg·mL -1 ) into the anesthetized mice by intraperitoneal injection, and injected the vaccine between the skins of the thighs of the mice in each group for a total of three inoculations with a time interval of three weeks. After the first vaccination, blood was collected from the mice one day before each vaccination, and the mice were sacrificed two weeks after the last vaccination and blood was collected to prepare cell-free serum and detect the titer of antigen-specific IgG by ELISA. The formulations of vaccines and adjuvants used in each group are shown in Table 1.
[0062] Table 1 Grouping and formulation of vaccine adjuvant dosage forms
[0063]
[0064] The IgG titer...
Embodiment 2
[0074] Embodiment 2: dose-dependent test
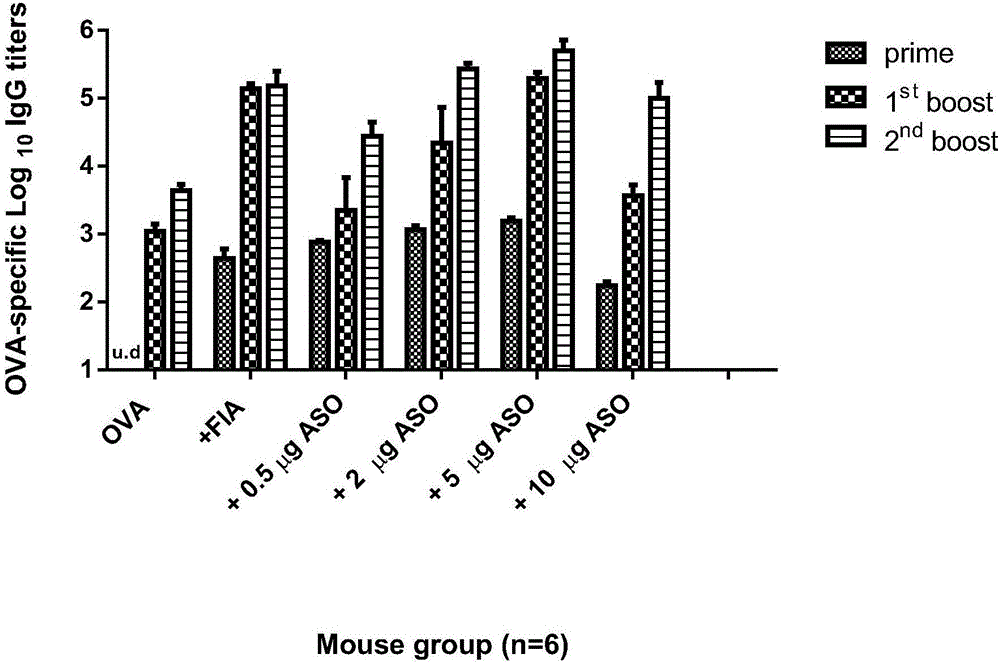
[0075] Test method: Select inbred strain Balb / c female mice without specific pathogens, aged 6-8 weeks, with a weight of 17g±0.5g, 36 were randomly divided into 6 groups, and the inoculation method and the determination of antibody IgG titer The method is the same as above. The vaccine adjuvant concentration groups and formulations of each group are shown in Table 2 below.
[0076] Table 2 Concentration grouping and formula of ASO adjuvant
[0077]
[0078]
[0079] The results of dose-dependent animal experiments such as image 3 As shown, when the dose of ASO was 5 μg per mouse, the highest antibody titer was produced, and the final concentration of ASO dose was 0.25 μg g -1 , that is, 0.25 μg g -1 In order to enhance the optimal adjuvant dose of vaccine immunogenicity, continuing to increase the adjuvant concentration cannot further improve the immune protection effect of the vaccine.
Embodiment 3
[0080] Embodiment 3: Cytotoxicity test of ASO
[0081] Mouse BMDC and human HeLa cell lines were selected for culture, and different concentrations of ASO, ASO control and PEI-B25 (a cationic polymer newly confirmed to have the function of an immune adjuvant, which has relatively low cytotoxicity) were added to the cell culture fluid. Strong, as a positive control group), cultured for 24h, 48h, and 72h, respectively, and carried out CCK-8 experiment to detect the cell survival rate of ASO, ASO control and PEI groups.
[0082] Experimental results such as Figure 4 with Figure 5 As indicated, the adjuvant concentration was 0.6 μg·mL -1 When , PEI, ASO, and ASO control did not show obvious toxicity, and the concentration was 4.8 μg·mL -1 , PEI began to exhibit cytotoxicity, and the cell survival rate began to decrease, and with the increase of the concentration, the stronger the toxicity of PEI, the lower the cell survival rate. However, the ASO and ASO control groups did n...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com