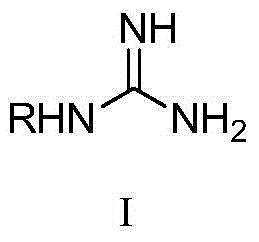
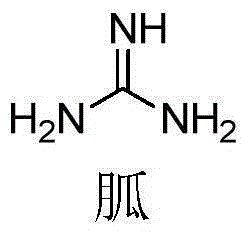
Application of guanidino compound
A compound, guanidine-based technology, applied in the application field of guanidine-based compounds, can solve problems such as toxic side effects, difficult synthesis, high cost, etc., and achieve remarkable effects and potential clinical application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
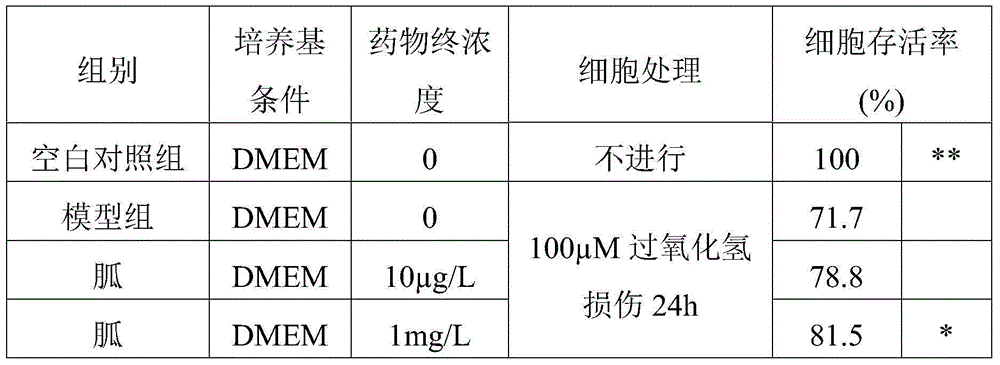
[0031] The effect of guanidine on the protective effect of 100 μM hydrogen peroxide-induced hippocampal nerve cell damage in neonatal rats was tested. The specific operation is: separate and culture the neurons in the hippocampal region of the rat brain born within 24 hours, and use 1x10 6 / ml cell density cultured for 7 days to the best state of growth. Then the well-grown hippocampal neurons were divided into blank group, model group and test drug group (n=4, ie 4 duplicate wells). The blank group and model group were given blank DMEM medium, and the treatment group was given medium containing different final concentrations of the drug to be tested. After 2h, add H 2 o 2 After 24 hours of injury in DMEM medium (final concentration: 100 μM), the cell viability was measured by MTT method. The results are shown in Table 1.
[0032] Table 1. Protective effect of guanidine on hippocampal nerve cell injury in neonatal rats induced by 100 μM hydrogen peroxide
[0...
Embodiment 2
[0039] Guanidine was tested for its inhibitory effect on Aβ aggregation in vitro. Since Aβ protein has the phenomenon of spontaneous aggregation in 10MPBS, the specific operation of this experiment is to set up different Aβ protein aggregation systems according to the experimental needs. Blank group: put in 10M PBS; Aβ 1-42Control group: Aβ dissolved in 10M PBS 1-42 , (Aβ 1-42 Final concentration 100μg / ml); Different drug groups to be tested: Aβ dissolved in 10M PBS 1-42 , and contain different concentrations of the drug to be tested (Aβ 1-42 The final concentration is 100 μg / ml), and the above Aβ protein aggregation system is put into a 96-well plate and cultured in a 37° C. incubator. Select the corresponding measurement time point. When measuring, take 2 μL of the solution of each aggregation system, add 198 μL of 3 μM Th-T (thioflavin T) to a black transparent bottom 96-well plate, set the microplate reader Ex=442nm, Em=480nm for detection, at this time Aβ 1-42 The f...
Embodiment 3
[0046]
[0047] According to the method of Example 2, the inhibitory effect of guanidine formic acid on Aβ aggregation in vitro was tested, and the results are shown in Table 3.
[0048] Table 3. Inhibitory effect of guanidine formic acid on Aβ aggregation in vitro (n=3, )
[0049]
[0050] Among them, * indicates that in the one-way analysis of variance, P<0.05, that is, the difference is significant compared with the model group; ** indicates that in the one-way analysis of variance, P<0.01, that is, the difference is extremely significant compared with the model group.
[0051] The level of effect in this example suggests that the compound may prevent and treat neurodegenerative diseases by inhibiting Aβ aggregation in the brain in future clinical applications.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com