Germacrone alcoholization and biological resolution method
A biological and rhododendron technology, applied in the field of rhododendron ketolization and bio-separation, can solve problems such as unseen and unseen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
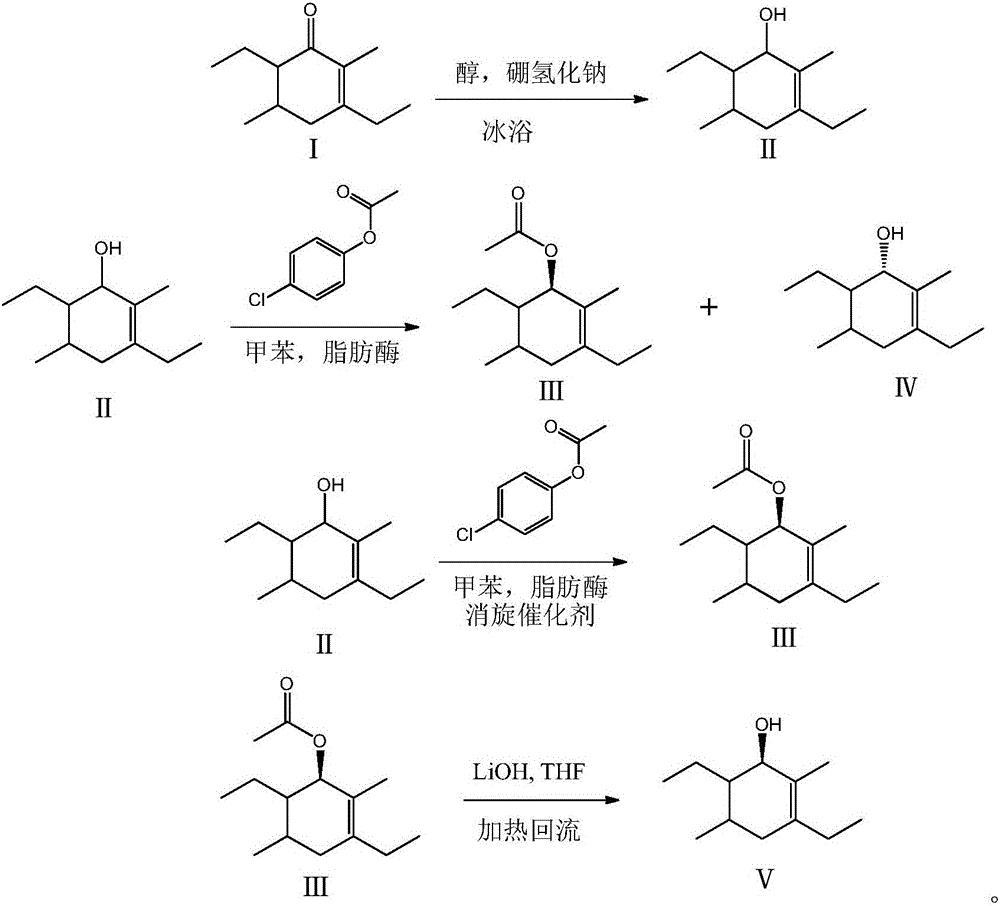
[0012] 1) At 0°C, add 250ml of anhydrous methanol and 18g of rhododendron into a single-necked flask. After stirring for 15 minutes, add 12g of sodium borohydride. After feeding, seal the flask with a balloon and keep it at 0°C for 3 hours. TLC detects Rhododendron The ketone reaction is complete, stop the reaction; quench sodium borohydride with dilute hydrochloric acid solution until no more bubbles emerge, distill off methanol and extract with 100ml dichloromethane three times, combine dichloromethane, dry and concentrate to obtain -3 , 17.8 g of 6-diethyl-2,5-dimethylcyclohex-2-en-1-ol, the yield was 98.0%.
[0013] 2) Add 60ml of toluene and 9.1g of 3,6-diethyl-2,5-dimethylcyclohex-2-en-1-ol in a constant temperature shaker with a 200ml blue cap bottle as the reaction vessel , 11g of p-chlorophenol acetate, 0.6g of porcine pancreatic lipase PPL, and 1.5g of acid resin D006. After feeding, the temperature was raised to 45°C for reaction. After 12 hours, -3,6-diethyl-2, ...
Embodiment 2
[0019] 1) At 0°C, add 1000ml of anhydrous methanol and 180g of rhododendron to a single-necked flask, stir for 20 minutes, then add 150g of sodium borohydride. After feeding, seal the flask with a balloon and keep it at 0°C for 4 hours. TLC detects rhododendron The ketone reaction is complete, stop the reaction; dilute the sodium borohydride with hydrochloric acid solution until there are no more bubbles, distill the methanol and extract with 300ml ethyl acetate three times, combine the ethyl acetate, dry and concentrate to get -3 , 178.9 g of 6-diethyl-2,5-dimethylcyclohex-2-en-1-ol, the yield was 98.3%.
[0020] 2) In a constant temperature shaker, with a 1000ml blue cap bottle as a reaction vessel, add 700ml of toluene, 91g of 3,6-diethyl-2,5-dimethylcyclohex-2-en-1-ol, 110g p-Chlorophenol acetate, 9g porcine pancreatic lipase PPL, 18g acid resin D006, after feeding, raise the temperature to 40°C for reaction, after 12 hours, detect-3,6-diethyl-2,5-dimethyl Cyclohexa-2-en...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com