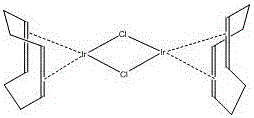
One-step synthesis method of (1,5-cyclooctadiene)-dichloro iridium dipolymer
A cyclooctadiene and dimer technology, applied in the field of preparation of precious metal catalysts, can solve the problems of long reaction time and harsh operation, and achieve the effects of low cost, improved yield and shortened reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: The preparation method of (1,5-cyclooctadiene)-iridium dichloride dimer comprises the following steps:
[0022] Weigh 2.5g (7.1 mmol) iridium trichloride hydrate, add it into a 100mL three-necked flask, repeatedly vacuumize and fill with nitrogen, and measure 50mL absolute ethanol, 0.88 mL of 1,5-cyclooctadiene (COD) was added to the reactor, and after stirring at room temperature to dissolve the solid, the reactor was transferred to an oil bath, and the reaction temperature was adjusted to 110°C, and kept The reaction temperature is heated to reflux reaction. During the reaction, the color of the solution gradually changes from dark brown to brick red. During the reaction, red crystals appear on the inner wall of the flask. After 6 hours of reaction, the reaction is stopped, cooled naturally to room temperature, and filtered under anaerobic operation. Reaction solution, then use a syringe to inject 35mL of ice-cold absolute ethanol to wash the solid in th...
Embodiment 2
[0024] Embodiment 2: the preparation method of (1,5-cyclooctadiene)-dichloroiridium dimer comprises the following steps:
[0025] Weigh 5.0g (14.2mmol) iridium trichloride hydrate, add it into a 250mL three-necked flask, repeatedly vacuumize and fill with nitrogen, and under the high-purity nitrogen atmosphere, measure 100mL of absolute ethanol, 2.11 mL of 1,5-cyclooctadiene (COD) was added to the reactor, and the solid was dissolved under stirring at room temperature, then the reactor was transferred to an oil bath, and the reaction temperature was adjusted to 120°C, and kept The reaction temperature is heated to reflux reaction. During the reaction, the color of the solution gradually changes from dark brown to brick red. During the reaction, red crystals appear on the inner wall of the flask. After 5 hours of reaction, the reaction is stopped, cooled naturally to room temperature, and filtered under anaerobic operation. Reaction solution, then use a syringe to inject 70mL o...
Embodiment 3
[0027] Embodiment 3: the preparation method of (1,5-cyclooctadiene)-iridium dichloride dimer comprises the following steps:
[0028] Take by weighing 7.5g (21.3mmol) iridium trichloride hydrate, join it in the three-necked flask of 500mL, repeatedly evacuate and fill with nitrogen, under the guarantee high-purity nitrogen atmosphere, measure successively 150mL dehydrated alcohol, 3.93 mL of 1,5-cyclooctadiene (COD) was added to the reactor, and the solid was dissolved under stirring at room temperature, then the reactor was transferred to an oil bath, and the reaction temperature was adjusted to 130°C, and kept The reaction temperature is heated to reflux reaction. During the reaction, the color of the solution gradually changes from dark brown to brick red. During the reaction, red crystals appear on the inner wall of the flask. After 4 hours of reaction, the reaction is stopped, cooled naturally to room temperature, and filtered under anaerobic operation. The reaction soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com